
Concept explainers
Interpretation:
The structures of the major organic products formed in the reaction of
Concept introduction:
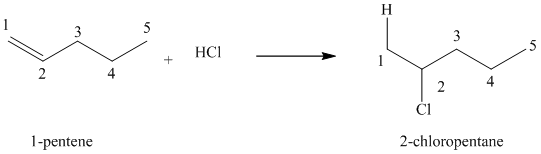
When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogens, and the halogen adds to the carbon that has fewer hydrogens. This rule is called Markovnikov’s rule.
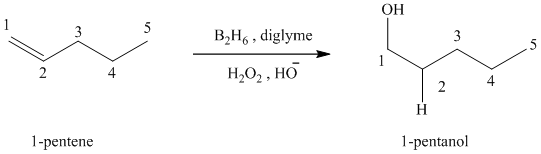
During hydroboration oxidation, hydrogen forms a bond with the carbon atom that has fewer hydrogens attached to it and the hydroxyl atom forms a bond with the carbon atom that has a greater number of hydrogens attached to it. This is a rule opposite to the Markovnikov’s addition.
Answer to Problem 28P
Solution:
Explanation of Solution
(a) Reaction of

The given alkene,
Hydrogen chloride gets added to the double bond of
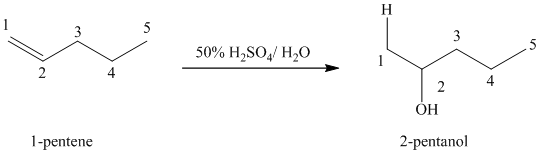
(b) Reaction of
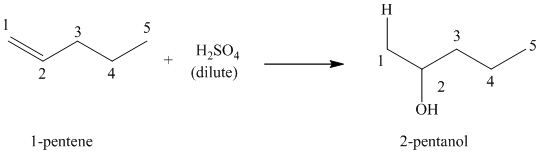
This reaction is an acid catalyzed electrophilic addition reaction of alkenes in which water molecule adds to the double bond in
A molecule of water adds to the double bond of
The addition mechanism for this reaction follows the Markovnikov’s rule. Therefore, the major organic product for the above acid-catalyzed electrophilic addition reaction is
(c) Reaction of
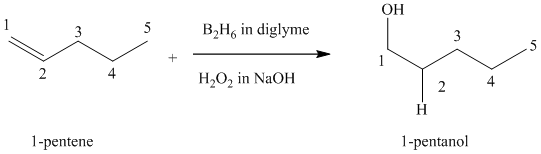
Hydroboration-oxidation leads to the overall hydration of an alkene. In hydroboration-oxidation,
The hydrogen atom in the water molecule adds to the carbon
In case of
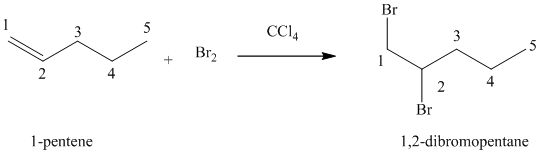
(d) Reaction of

Bromine reacts rapidly with alkenes by electrophilic addition. The products are called vicinal dibromides, meaning that the bromine atoms get attached to adjacent double bonded carbon atoms. It is carried out in suitable solvents like
A molecule of bromine adds across the double bond in
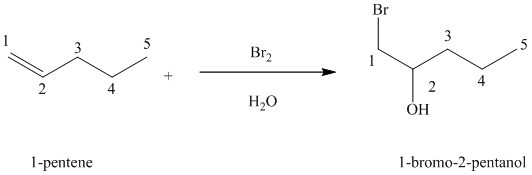
(e) Reaction of
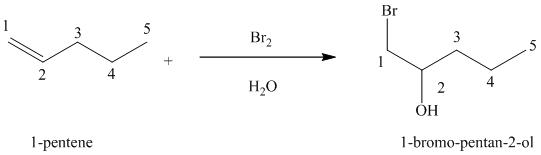
Chlorine and bromine react with alkenes in aqueous solution to give the corresponding vicinal halohydrins – compounds that add a halogen and hydroxyl group on adjacent carbon atoms in the alkene. The halogen atom forms a bond with that carbon atom in alkene, which has a greater number of hydrogen atoms, while the hydroxyl group bonds to that carbon atom in alkene, which has a fewer number of hydrogen atoms.
In the reaction of
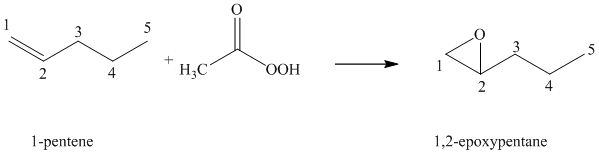
(f) Reaction of
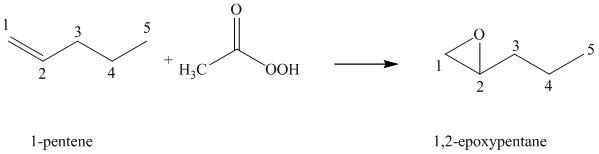
Peroxyacid transfers oxygen to the double bond of alkene to yield epoxides, which is a three-membered oxygen-containing ring.
When
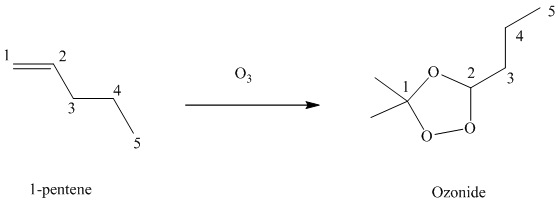
(g) Reaction of
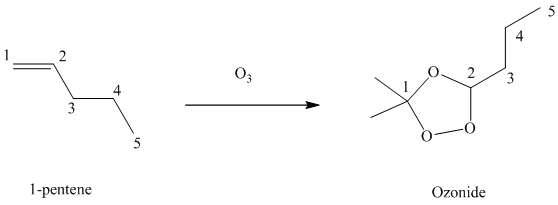
Ozone is a powerful electrophile and reacts with alkenes to cleave the double bond between two oxygen atoms in the molecule, forming an ozonide.
When
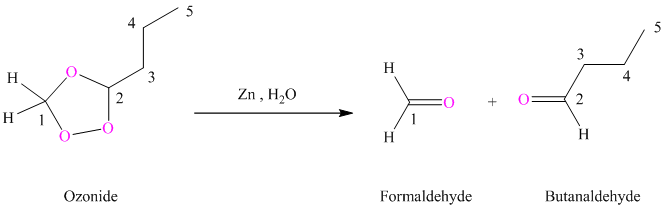
(h) Product of part (g) treated with zinc in water
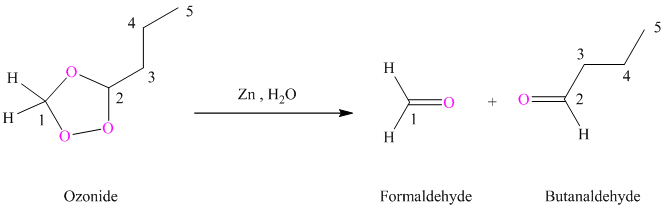
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water giving carbonyl compounds. Depending upon the structure of the starting alkene, various carbonyl compounds such as formaldehyde, aldehydes, or ketones are formed.
When corresponding ozonide of
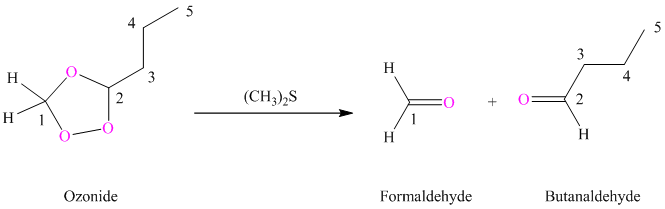
(i) Product of part (g) is treated with dimethyl sulfide.
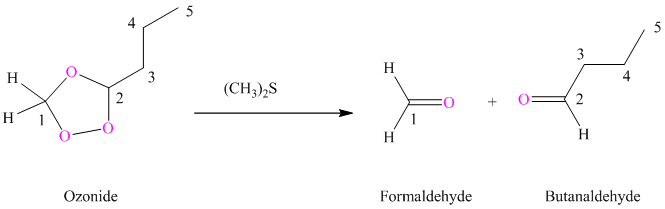
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water, giving carbonyl compounds. Depending upon the structure of the starting alkene, various carbonyl compounds such as formaldehyde, aldehydes, or ketones are formed.
When corresponding ozonide of
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





