
Concept explainers
Oxymercuration
Concerns about mercury’s toxicity have led to decreased use of mercury-based reagents in
Among the synthetically useful reactions of
salts with organic compounds, the most familiar is a two-stage procedure for alkene hydration called oxymercuration–demercuration. Its application in the conversion of
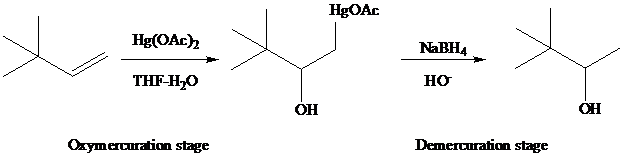
The reaction is performed in two operations, the first of which is oxymercuration. In this stage the alkene is treated with
of the alkene. The oxygen of water, one of the components in the
solvent mixture, bonds to
to
From the overall reaction, we see that oxymercuration–demercuration
1. accomplishes hydration of the double bond in accordance with Markovnikov’s rule, and
2. carbocation rearrangements do not occur.
Additional information from stereochemical studies with other
3. anti addition of
and
characterizes the oxymercuration stage, and
4. the replacement of
c by H in the demercuration stage is not stereospecific.
The structure of the intermediate in oxymercuration has received much attention and can be
approached by considering what is likely to happen when the electrophile
reacts with the double bond of an alkene.
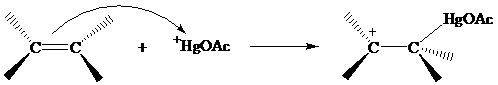
Recall from Section
that electrons in bonds that are
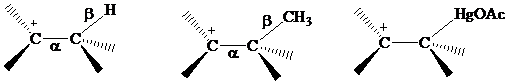
The electrons in a
or
electrons, making
stabilization by hyperconjugation more effective for
or
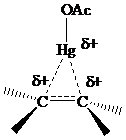
The problems that follow explore various synthetic aspects of oxymercuration–demercuration.
Experimental procedures sometimes vary depending on the particular transformation. The source of the electrophile may be a mercury(II) salt other than
Oxymercuration–demercuration of allyl alcohol gives
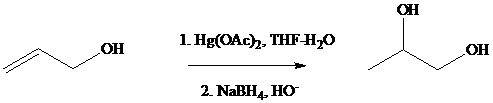
Under the same conditions, however,
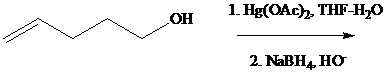
What is the most reasonable structure for the product of this reaction?
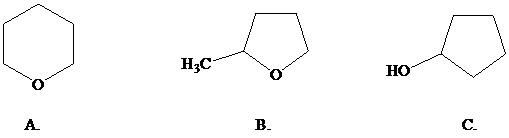
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Provide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forward
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


