
Concept explainers
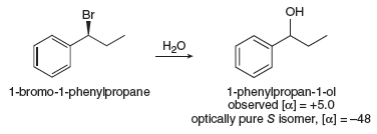
In some nucleophilic substitutions under
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry-Package(Custom)
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
General, Organic, and Biological Chemistry - 4th edition
Biology: Concepts and Investigations
Essentials of Human Anatomy & Physiology (12th Edition)
- S: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardResults Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward
- 301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forwardConsider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forward
- Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forwardStarting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward- The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward
- 15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forwardDraw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forwardChoose the best reagent(s) for carrying out the following conversions from the list below. Place the letter corresponding to the best choice in the blank to the left of the conversion. a. KMnO4, H3O+ b. Tollens' Reagent [oxidizing reagent] C. NaBH4, ethanol d. 1. BH3 2. H3O+ e. 1. CH3MgBr, ether 2. H3O+ f. CrO3, H2SO4, H₂O g. 1. Mg, ether 2. CO2 3. H3O+ h. 1. NaCN 2. H2SO4, H2, heat i. O3, then Zn and HOAC j. CH₂I A. B. C. CH CH=CHCH2COOH Br CEN CH COOH + HOOCCH COOH COOH 010 CH3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
