
Concept explainers
Light Energy, and the Hydrogen Atom
- a Which has the greater wavelength, blue light or red light?
- b How do the
frequencies of blue light and red light compare? - c How does the energy of blue light compare with that of red light?
- d Does blue light have a greater
speed than red light ?- e How does the energy of three photons from a blue light source compare with the energy of one photon of blue light from the same source? How does the energy of two photons corresponding to a wavelength of 451 nm (blue light) compare with the energy of three photons corresponding to a wavelength of 704 nm (red light)?
- f A hydrogen atom with an electron in its ground state interacts with a photon of light with a wavelength of 1.22 × 10−6 m. Could the electron make a transition from the ground state to a higher energy level? If it does make a transition, indicate which one. If no transition can occur, explain.
- g If you have one mole of hydrogen atoms with their electrons in the n = 1 level, what is the minimum number of photons you would need to interact with these atoms in order to have all of their electrons promoted to the n = 3 level? What
wavelength of light would you need to perform this experiment?
(a)
Interpretation:
The light with higher wavelength has to be identified from the given lights.
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength
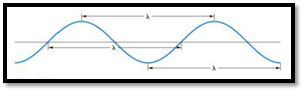
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Answer to Problem 7.23QP
Red light has larger wavelength than blue light.
Explanation of Solution
To identify: The light with higher wavelength.
Wavelength and frequency are inversely proportional to each other. Frequency of blue light is higher than red light. Thus, red light has larger wavelength than blue light.
By using the relation between wavelength and frequency, the light with higher wavelength was identified.
(b)
Interpretation:
The light with higher frequency has to be identified from the given lights.
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength
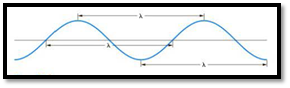
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Answer to Problem 7.23QP
Blue light has larger frequency than red light.
Explanation of Solution
To identify: The light with higher frequency.
Wavelength and frequency are inversely proportional to each other. Wavelength of blue light is less than red light. Thus, blue light has larger frequency than red light.
By using the relation between wavelength and frequency, the light with higher frequency was identified.
(c)
Interpretation:
The energies of blue light and red light has to be related.
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength

Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Answer to Problem 7.23QP
Blue light has higher energy than red light.
Explanation of Solution
To compare: The energy of blue light and red light.
Energy and frequency are directly proportional to each other. Frequency of blue light is higher than red light. Thus, blue light has higher energy than red light.
By using the relation between wavelength and frequency, the energy of blue light and red light was related.
(d)
Interpretation:
The speed of blue light and red light has to be compared.
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength
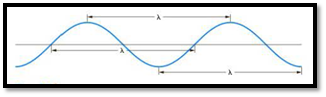
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Answer to Problem 7.23QP
Blue light has higher energy than red light.
Explanation of Solution
To compare: The speed of blue light and red light.
Energy and frequency are directly proportional to each other. Frequency of blue light is higher than red light. Thus, blue light has higher energy than red light.
By using the relation between wavelength and frequency, the energy of blue light and red light was related.
(e)
Interpretation:
The energy of one photon and energy of three photons of blue light has to be compared.
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength
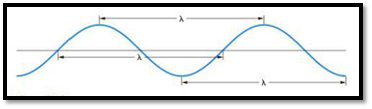
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Answer to Problem 7.23QP
Blue light (
Explanation of Solution
To compare: The energy of one photon and energy of three photons of blue light.
Blue light with energy of three photons is thrice the energy of one photon. In order to relate the energy of two photon (blue light) with energy of three photon (red light), the energy is given as
Blue light (
By using the relation between wavelength and frequency, the energy of two photon of blue light and energy of three photons of red light was related.
(f)
Interpretation:
The possibility of transition of an electron of hydrogen atom from ground state to higher energy state has to be determined.
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength
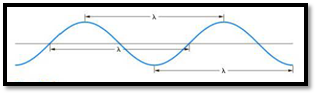
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Bohr developed a rule for quantization of energy that could be applicable to the electron of an atom in motion. By using this he derived a formula for energy levels of electron in H-atom.
Answer to Problem 7.23QP
The obtained energy for transition is greater than the energy of light. Therefore, no transition occurs.
Explanation of Solution
To determine: The possibility of transition of an electron of hydrogen atom from ground state to higher energy state.
By using the below formula and calculate frequency and energy of light
The minimum energy required for the transition of
The obtained energy for transition is greater than the energy of light. Therefore, no transition occurs.
The possibility of transition of an electron of hydrogen atom from ground state to higher energy state was determined.
(g)
Interpretation:
The energy required for transition of an electron of hydrogen atom from
Concept introduction:
Relation between frequency and wavelength is,
C is the speed of light.
h is Planck’s constant (
E is energy of light particle.
Wavelength and frequency are inversely proportional to each other.
The distance between any two similar points of a wave is called wavelength
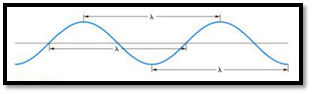
Figure 1
Frequency is defined as number of wavelengths of a wave that can pass through a point in one second.
Bohr developed a rule for quantization of energy that could be applicable to the electron of an atom in motion. By using this he derived a formula for energy levels of electron in H-atom.
Answer to Problem 7.23QP
The minimum energy required for the transition of
The wavelength of the light is
Explanation of Solution
To determine: The energy required for transition of an electron of hydrogen atom from
The minimum energy required for the transition of
The energy required for transition of an electron of hydrogen atom from
Want to see more full solutions like this?
Chapter 7 Solutions
General Chemistry - Standalone book (MindTap Course List)
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





