
Concept explainers
(a)
Interpretation:
The hybridization and approximate bond angle for each carbon atoms in
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
(a)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for alanine is given below:
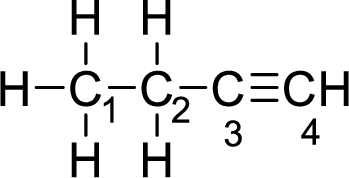 .
.
Consider the first carbon. There will be four electron regions in the molecule and hence the electron-region geometry will be tetrahedral. For a molecule having tetrahedral geometry, the hybridization will be
Consider the second carbon. There will be four electron regions in the molecule and hence the electron-region geometry will be tetrahedral. For a molecule having tetrahedral geometry, the hybridization will be
Consider the third carbon. There will be two electron regions in the molecule and hence the electron-region geometry will be linear. For a molecule having linear geometry, the hybridization will be
Consider the fourth carbon atom. There will be two electron regions in the molecule and hence the electron-region geometry will be linear. For a molecule having linear geometry, the hybridization will be
(b)
Interpretation:
The shortest carbon-to-carbon bond length in molecule has to be identified.
Concept Introduction:
Bond length is the distance between the nuclei in a bond and it is related to the sum of the covalent radii at the bonded atoms.
Higher the bond order, shorter will be the bond length.
(b)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for alanine is given below:
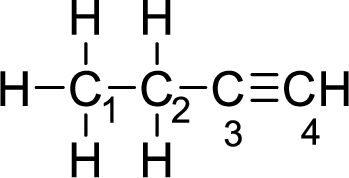 .
.
In the given molecule, the triple bond is present between
(c)
Interpretation:
The strongest carbon-to-carbon bond length in molecule has to be identified.
Concept Introduction:
Comparing triple bond, double bond and single bond, the bond with higher energy is the triple bond.
(c)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for alanine is given below:
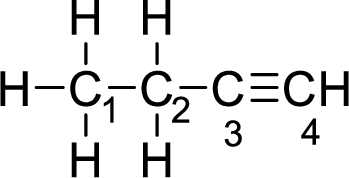 .
.
In the given molecule, the triple bond is present between
The strength of a triple bond is more than the single bond.
Thus, the strongest carbon-to-carbon bond is between
Want to see more full solutions like this?
Chapter 7 Solutions
OWLV2 FOR MOORE/STANITSKI'S CHEMISTRY:
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


