
Concept explainers
(a)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
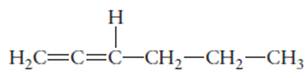
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
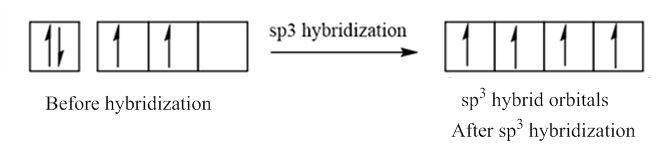
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(b)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
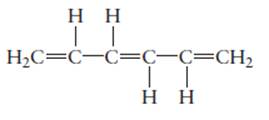
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
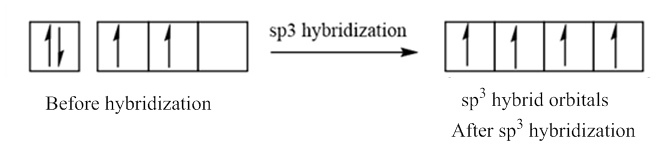
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(c)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
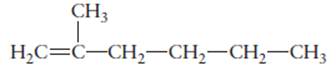
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
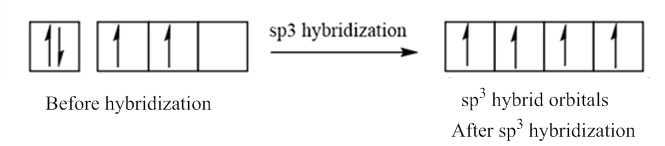
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(d)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
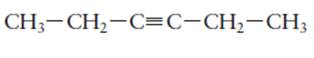
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
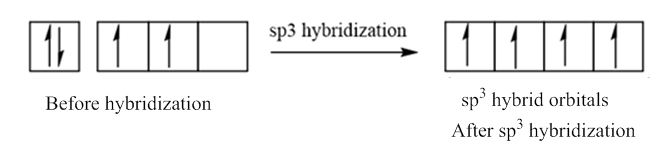
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Principles of Modern Chemistry
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





