
Concept explainers
(a)
Interpretation:
From the given compounds, the compound with high regioselective ability towards addition of
Concept introduction:
Addition Reaction: It is defined as
The product of electrophilic addition reaction obtained by addition of electrophile to
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
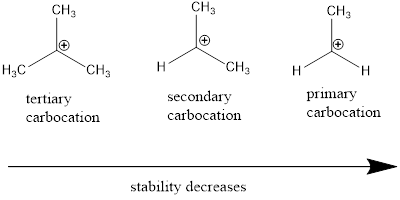
Cation: The positively charged chemical species is referred as cation.
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
(b)
Interpretation:
From the given compounds, the compound with high regioselective ability towards addition of
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
The product of electrophilic addition reaction obtained by addition of electrophile to
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
Cation: The positively charged chemical species is referred as cation.
Regioselective reaction: They are reactions which contain more than one product which are actually molecules with same molecular formula but different in the way they are connected and among those products only one product is major.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
- 11) The Ksp expression for copper (II) sulfate is: a. [Cu2+][SO4²¯] b. [Cu²+]² [SO4²]² c. [Cu²+]²[SO4²] d. [CuSO4] 12) Which of the following is true about a chemical system in equilibrium? a. All chemical reactions have stopped b. The concentration of reactants is equal to the concertation of products c. The forward and reverse reaction rates become equal d. The system will remain at equilibrium regardless of any external factorsarrow_forward21) Explain the difference between the rate of a reaction and the extent of a reaction. Why are both of these concepts important, if you are a chemical engineer that is trying to develop a process to produce a large volume of a specific type of chemical compound?arrow_forwardPls help.arrow_forward
