
(a)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to
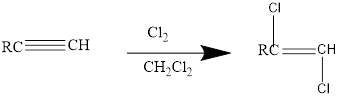
If excess halides are present, a second addition reaction also occurs.
(b)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine add to the alkyne compounds. The general form of the reaction is given below.
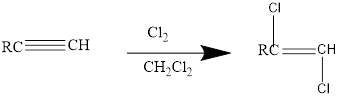
If excess halides are present, a second addition reaction also occurs.
(c)
Interpretation:
The major product obtained from the reaction of given compound with excess of
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine add to the alkyne compounds. The general form of the reaction is given below.
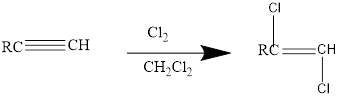
If excess halides are present, a second addition reaction also occurs.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
