
Concept explainers
(a)
Interpretation:
The more stable compound from the given set of compounds should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:
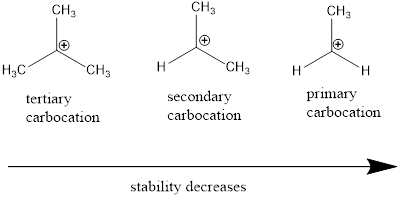
Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
(b)
Interpretation:
The more stable compound from the given set of compounds should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:

Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
(c)
Interpretation:
The more stable compound from the given set of compounds should be determined.
Concept introduction:
Carbocation: It is carbon ion that bears a positive charge on it.
Leaving group: It is a fragment that leaves from a substrate with a pair of electrons via
Carbocation stability order:

Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardDraw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forward
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardPls help.arrow_forward
- 13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forwardPls help.arrow_forward
- 16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward11) The Ksp expression for copper (II) sulfate is: a. [Cu2+][SO4²¯] b. [Cu²+]² [SO4²]² c. [Cu²+]²[SO4²] d. [CuSO4] 12) Which of the following is true about a chemical system in equilibrium? a. All chemical reactions have stopped b. The concentration of reactants is equal to the concertation of products c. The forward and reverse reaction rates become equal d. The system will remain at equilibrium regardless of any external factorsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning


