
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.11, Problem 29P
What products are formed when the following compounds react with ozone and then with dimethyl sulfide?
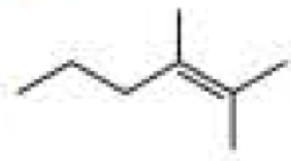
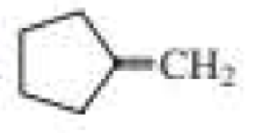
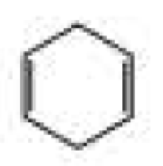
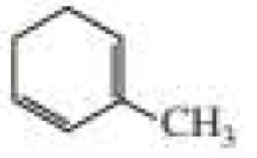
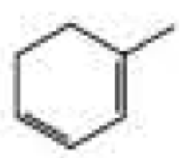
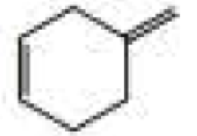
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Just try completing it and it should be straightforward according to the professor and TAs.
The grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.
Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!
Chapter 6 Solutions
Organic Chemistry
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are avilable for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.4 - Prob. 6PCh. 6.4 - What alkene should be used to synthesize each of...Ch. 6.4 - Prob. 8PCh. 6.5 - The pKa of a protonated alcohol is about 2.5, and...Ch. 6.5 - Prob. 10P
Ch. 6.5 - Prob. 11PCh. 6.6 - a. What is the major product or each or the...Ch. 6.6 - Prob. 14PCh. 6.6 - Prob. 15PCh. 6.7 - What is the major product obtained from the...Ch. 6.8 - Which is more highly regionselective: reaction of...Ch. 6.8 - Prob. 19PCh. 6.9 - What will be the product of the preceding reaction...Ch. 6.9 - Prob. 21PCh. 6.9 - Prob. 22PCh. 6.9 - Prob. 23PCh. 6.9 - What is the product of the addition of 1Cl to...Ch. 6.9 - What will be the major product obtained from the...Ch. 6.9 - Propose a mechanism for the following reaction:Ch. 6.10 - Draw structures for the following: a. 24...Ch. 6.10 - What alkene would you treat with a peroxyacid in...Ch. 6.11 - What products are formed when the following...Ch. 6.11 - Prob. 30PCh. 6.11 - Prob. 31PCh. 6.12 - Prob. 34PCh. 6.12 - Prob. 35PCh. 6.13 - Prob. 36PCh. 6.13 - Prob. 37PCh. 6.13 - Prob. 38PCh. 6.14 - What characteristics must the reactant of a...Ch. 6.15 - Prob. 40PCh. 6.15 - What stereoisomers are obtained from each of the...Ch. 6.15 - Prob. 45PCh. 6.15 - Prob. 46PCh. 6.15 - Prob. 47PCh. 6.15 - Prob. 49PCh. 6.15 - Prob. 50PCh. 6.15 - Prob. 51PCh. 6.15 - Prob. 52PCh. 6.15 - Prob. 53PCh. 6.16 - Prob. 54PCh. 6.17 - Prob. 55PCh. 6.18 - Explain why 3-methykyclohexene should not be used...Ch. 6.18 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - What is the major product of the reaction of...Ch. 6 - Give two names for each of the following:Ch. 6 - Prob. 63PCh. 6 - Prob. 64PCh. 6 - Prob. 65PCh. 6 - What are the products of the following reactions?...Ch. 6 - When 3-methyl-1-butene reacts with HBr, two alkyl...Ch. 6 - Prob. 68PCh. 6 - Draw curved arrows to show the flow of electrons...Ch. 6 - What reagents are needed to carry out the...Ch. 6 - Prob. 71PCh. 6 - Prob. 72PCh. 6 - Prob. 73PCh. 6 - Prob. 74PCh. 6 - a. Draw the product or products that will be...Ch. 6 - The second-order rate constant (in units of M1s1)...Ch. 6 - Which compound has the greater dipole moment?Ch. 6 - Prob. 78PCh. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83PCh. 6 - Prob. 84PCh. 6 - Prob. 85PCh. 6 - Prob. 86PCh. 6 - Prob. 87PCh. 6 - Prob. 88PCh. 6 - Prob. 89PCh. 6 - Prob. 90PCh. 6 - 91. a. How many alkenes could you treat with H2,...Ch. 6 - Draw the products of the following reactions. If...Ch. 6 - Prob. 93PCh. 6 - Prob. 94PCh. 6 - Two chemists at Dupont found that lCH2Znl is...Ch. 6 - Prob. 96PCh. 6 - Prob. 97PCh. 6 - What alkene gives the product shown after...Ch. 6 - Prob. 99PCh. 6 - Prob. 100PCh. 6 - Prob. 101PCh. 6 - Prob. 102PCh. 6 - Prob. 103PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 106PCh. 6 - Prob. 107P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forwardPredict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward
- 70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forwardShow your steps. Hopefully, you get everything correctly or a reasonable guess that is close to the correct answer.arrow_forward
- Please give it your best shot at answering this question.arrow_forwardLook the image attaarrow_forwardPart C: Communication (/9) 17. Compare and contrast the Thomson, Rutherford and Bohr models of the atom using the chart below. You can use words and/or diagrams in your answers. (9) What was the experiment that led to the model? Where is positive charge in the atom located in the model? Where are electrons located in the molecule? Thomson Model Rutherford Model Bohr Model 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning 
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY