
Concept explainers
(a)
Interpretation:
The mechanism for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
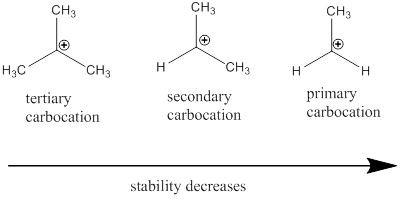
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
(b)
Interpretation:
The rate determining step for the given reaction should be determined.
Concept introduction:
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Rate determining step: The slowest step in the reaction is called rate determining step. The actual
(c)
Interpretation:
The electrophile present in the first step of given reaction should be determined.
Concept introduction:
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
(d)
Interpretation:
The nucleophile present in the first step of given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
(e)
Interpretation:
The electrophile present in the second step of given reaction should be determined.
Concept introduction:
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
(f)
Interpretation:
The nucleophile present in the second step of given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- How many products are possible from the following reaction? Do not take into account stereoisomers. 01 04 03 O O O O 02 CH H₂SO4 heatarrow_forwardplease helparrow_forwardChoose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forward
- Select the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forwardWhat is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forward
- Indicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forwardWhat are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardThe following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
