
To review:
The type of inhibition by A and B inhibitor and the kind of affinity they possess for ES versus E.
Introduction:
Enzyme inhibitors are the molecules that are able to bind to enzyme and reduce the activity. Enzyme inhibitors work by several ways such as preventing the substrate from entering the active site of an enzyme or hinder in catalyzing the reaction. There are three types of enzyme inhibitors, namely, competitive, noncompetitive, and uncompetitive.
Explanation of Solution
The velocity of the reaction at different substrate concentrations in presence of A inhibitor and B inhibitor are given in the question. For calculating, the Vmax (maximum
| [S] in mM (millimolar) |
V | |
|
| 1.3 | 1.17 | 0.76 | 0.855 |
| 2.6 | 2.10 | 0.38 | 0.476 |
| 6.5 | 4.00 | 0.15 | 0.25 |
| 13.0 | 5.70 | 0.077 | 0.175 |
| 26.0 | 7.20 | 0.038 | 0.139 |
The following table shows the values of [S], V,
| [S] in mM (millimolar) |
V | |
|
| 1.3 | 0.62 | 0.76 | 1.613 |
| 2.6 | 1.42 | 0.38 | 0.704 |
| 6.5 | 2.65 | 0.15 | 0.377 |
| 13.0 | 3.12 | 0.077 | 0.321 |
| 26.0 | 3.58 | 0.038 | 0.28 |
Plotting the graph between
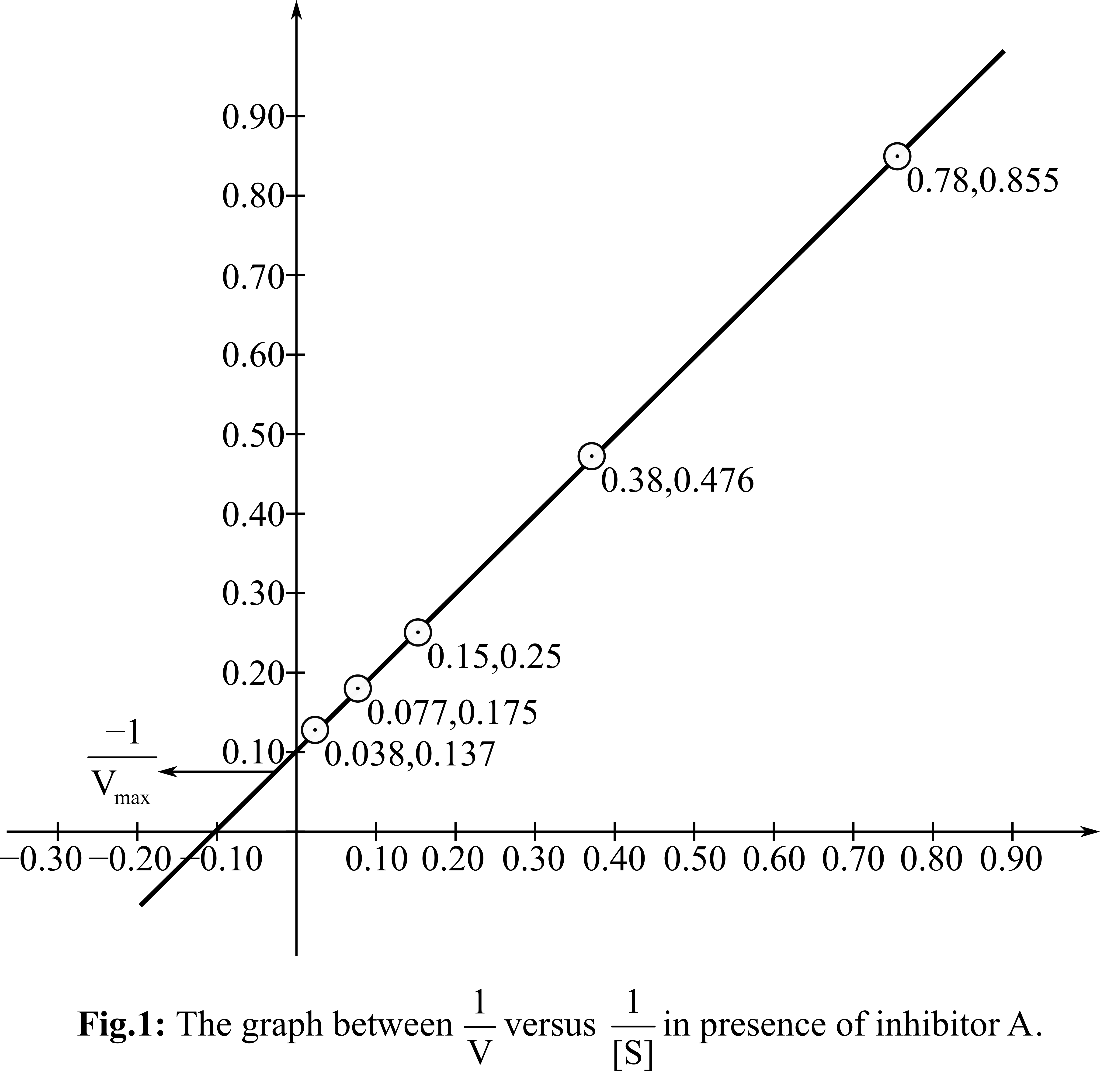
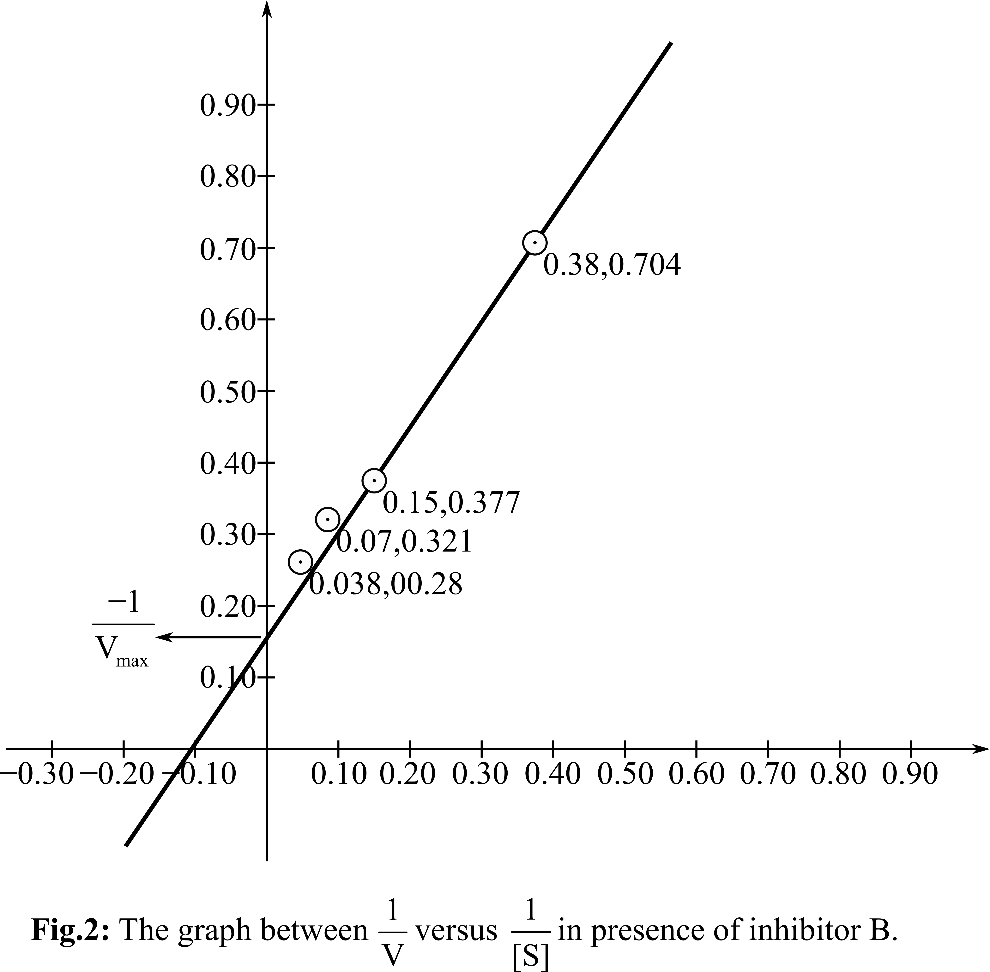
According, to the Michelis Menten equation, the value of y-intercept represents
From the graph, it can be observed that the value of y-intercept is 0.10 mM-1 sec. y-Intercept represents inverse of Vmax.
KM can be calculated by putting the value of Vmax in the equation Slope=KM/Vmax
So, the KM for the reaction is 9.8and the Vmax is 10 mM. sec-1. In presence of inhibitor A, KM is changed but Vmax is unchanged. So, it is a competitive inhibitor.
According, to the Michelis Menten equation, the value of y-intercept represents
From the graph, it can be observed that the value of y-intercept is 0.25 mM-1 sec. y-Intercept represents inverse of Vmax.
KM can be calculated by putting the value of Vmax in the equation Slope=KM/Vmax
So, the KM for the reaction is 5.68 and the Vmax is4 mM. sec-1. In presence of inhibitor B, KM andVmax both are changed. So, it is anuncompetitive inhibitor. A inhibitor is a competitive inhibitor so it can bind to free enzyme and has no affinity for ES. B inhibitor is an uncompetitive inhibitor, so it can bind to ES as well as E.
Therefore, it can be concluded that inhibitor A is competitive as Vmax remain unchanged whereas, inhibitor B is uncompetitive inhibitor. A can bind to free enzyme and B can bind to E as well as ES.
Want to see more full solutions like this?
Chapter 6 Solutions
Biochemistry: The Molecular Basis of Life
- answer the questions and the example steps should be from carbohydrates glycolysis and citric acid cycle. Please put down reactions and structuresarrow_forwardidentify the general type of reaction catalyzed and an example step from glycolisis structure for each of the following enzymes/ co factor Kinase, isomerase, mutase, dehydrogenase, NAD+ , FADarrow_forwardfill in the blanks with the missing structures and give namesarrow_forward
- fill in the table and identify the general type of reaction catalayzed and an example step from the structures in the second page so you will answer the questions from the first page the second one is just a reference urgently!arrow_forwardPlease draw out the molecular structures of each molecule and show how each enzyme + cofactor would affect the following molecule in the human metabolic pathway. (This is a metabolic map)arrow_forwardPlease draw out the molecular structures of each molecule and show how an enzyme + cofactor would affect the following molecule in the human metabolic pathway to create energy.arrow_forward
- Please draw out the molecular structures of each molecule and show how each enzyme + cofactor would affect the following molecule in the human metabolic pathway.arrow_forwardPlease draw out the mechanism with curved arrows showing electron flow. Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide the mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardPyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide the mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forward
- The mitochondrial ATP synthase has 10 copies of the F0 subunit “c”, and the [H ] in the mitochondrial inner membrane space (IMS) is 6.31 x 10-8 M and the [H + ] in the matrix is 3.16 x 10-9 M. Calculate the minimum membrane potential (∆Ψ) necessary to make ATP synthesis thermodynamically favorable. [Assume ∆G' ofphosphate hydrolysis of ATP is - 45 kJ/mol.]arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency in any one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and is characterized by cardiac and neurological symptoms. One key diagnostic for this disease is an increased level of pyruvate and α-ketoglutarate in the bloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patient suffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardPyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning





