
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
16th Edition
ISBN: 9781323406038
Author: McMurry
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.65CP
Acetylsalicylic acid, the active ingredient in aspirin, is prepared from salicylic acid by reaction with acetic anhydride.
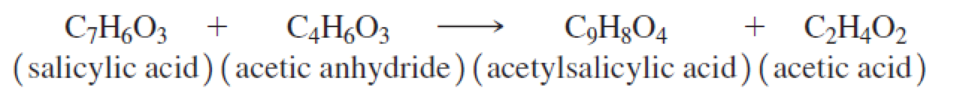
- (a) Calculate the theoretical yield if 47 g of salicylic acid is reacted with 25 g of acetic anhydride.
- (b) What is the percent yield if only 35 g is obtained?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.
Please sketch the structure and use arrows showing electron flow!
I am a Biochemistry student and I am confused on how to analyze FRAP Analysis using Excel Spread Sheets.
The following spread sheet has my 0 minute data listed at top and the 4 minute data listed on the bottom.
Sheet: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EXjrCizWiXRPmpittqZA12IB8EkB5eE8iaRqj_iun-IAtg?rtime=Wo9zPHFY3Ug
The formula for FRAP Analysis is:
FRAP value = A (4 min sample) - A (0 min sample) over A (4 min 30 uM ascorbic acid) - A (0 min 30 uM ascorbic acid) multiplied by 30 uM and the dilution factor of 1/10
HO
Fill in the missing boxes.
ON
800
NO
NO
Glucose
ATP
NADH
Hexokinase
1,3-Bisphosphoglycerate
Mg2+
ADP
NAD+, Pi
Phosphoglucose
Isomerase
Glucose-6-Phosphate
ON
沁
Glyceraldehyde-3-Phosphate
HO
حلمة
ADP
ADP Phospho
Mg2+
glycerate
Dihydroxyacetone Phosphate
ATP
kinase
ATP
Phosphoglycerate
3-phosphoglycerate
Mutase
H₁₂O
Fructose-6-Phosphate
ATP
Mg2+
ADP
Fructose-1,6-Bisphosphate
2-phosphoglycerate
H₂O
Phosphoenolpyruvate
ADP
Mg2+
ATP
Pyruvate
Chapter 6 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
Ch. 6.1 - Calculate the molecular weight of the following...Ch. 6.1 - How many molecules of ascorbic acid (vitamin C,...Ch. 6.1 - What is the mass in grams of 5.0 1020 molecules...Ch. 6.1 - What is the molecular weight of cytosine, a...Ch. 6.2 - Prob. 6.5PCh. 6.2 - Prob. 6.6PCh. 6.2 - Prob. 6.7PCh. 6.3 - Prob. 6.8PCh. 6.3 - Prob. 6.9PCh. 6.4 - Hydrogen fluoride is one of the few substances...
Ch. 6.4 - The tungsten metal used for filaments in light...Ch. 6.5 - What is the theoretical yield of ethyl chloride in...Ch. 6.5 - The reaction of ethylene oxide with water to give...Ch. 6.5 - Prob. 6.14KCPCh. 6.5 - Dietary iron forms a 1:1 complex with hemoglobin...Ch. 6.5 - Prob. 6.2CIAPCh. 6.5 - Prob. 6.3CIAPCh. 6 - Methionine, an amino acid used by organisms to...Ch. 6 - Prob. 6.16UKCCh. 6 - Prob. 6.17UKCCh. 6 - Prob. 6.18UKCCh. 6 - Prob. 6.19UKCCh. 6 - Prob. 6.20APCh. 6 - What is the difference between molecular weight...Ch. 6 - Prob. 6.22APCh. 6 - Prob. 6.23APCh. 6 - How many calcium atoms are in 16.2 g of calcium?Ch. 6 - What is the mass in grams of 2.68 1022 atoms of...Ch. 6 - Calculate the molar mass of each of the following...Ch. 6 - Prob. 6.27APCh. 6 - Prob. 6.28APCh. 6 - Caffeine has the formula C8H10N4O2. If an average...Ch. 6 - How many moles of aspirin, C9H8O4, are in a 500 mg...Ch. 6 - What is the molar mass of diazepam (Valium),...Ch. 6 - Calculate the molar masses of the following...Ch. 6 - How many moles are present in a 4.50 g sample of...Ch. 6 - How many grams are present in a 0.075 mol sample...Ch. 6 - The principal component of many kidney stones is...Ch. 6 - Prob. 6.36APCh. 6 - Ethyl acetate reacts with H2 in the presence of a...Ch. 6 - Prob. 6.38APCh. 6 - Ammonia, NH3, is prepared for use as a fertilizer...Ch. 6 - Hydrazine, N2H4, a substance used as rocket fuel,...Ch. 6 - Prob. 6.41APCh. 6 - Magnesium metal burns in oxygen to form magnesium...Ch. 6 - Titanium metal is obtained from the mineral...Ch. 6 - Prob. 6.44APCh. 6 - Prob. 6.45APCh. 6 - Prob. 6.47APCh. 6 - Prob. 6.48APCh. 6 - Once made by heating wood in the absence of air,...Ch. 6 - In Problem 6.40, hydrazine reacted with oxygen...Ch. 6 - Dichloromethane, CH2Cl2, the solvent used to...Ch. 6 - Cisplatin [Pt(NH3)2Cl2], a compound used in cancer...Ch. 6 - Prob. 6.53APCh. 6 - Prob. 6.54APCh. 6 - Prob. 6.55CPCh. 6 - Prob. 6.56CPCh. 6 - Prob. 6.57CPCh. 6 - Prob. 6.58CPCh. 6 - Prob. 6.59CPCh. 6 - Prob. 6.60CPCh. 6 - Gaseous ammonia reacts with oxygen in the presence...Ch. 6 - Sodium hypochlorite, the primary component in...Ch. 6 - Barium sulfate is an insoluble ionic compound...Ch. 6 - The last step in the production of nitric acid is...Ch. 6 - Acetylsalicylic acid, the active ingredient in...Ch. 6 - Jewelry and tableware can be silver-plated by...Ch. 6 - Elemental phosphorus exists as molecules of P4. It...Ch. 6 - Lithium oxide is used aboard the International...Ch. 6 - Prob. 6.69CPCh. 6 - Prob. 6.70GPCh. 6 - Obtain a bottle of aspirin and identify the amount...Ch. 6 - Lovastatin, a drug used to lower serum...Ch. 6 - Pyrite, also known as fools gold, is used...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- In a diffraction experiment of a native crystal, intensity of reflection (-1 0 6) is equivalent to the intensity of reflection (1 0 -6). true or false?arrow_forwardin an x-ray diffraction experiment, moving the detector farther away from the crystal will allow collection of reflection of reflections with high Miller indices. true or false?arrow_forwardShow the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.arrow_forward
- Label the following polysaccharide derivatives as reducing or nonreducing. a. C. b. HO CH₂OH CH2OH OH OH OH OH OH HOCH₂ OH OH OH HOCH₂ HO HO HO OH OH ΙΟ CH₂OH OH OH "OH OHarrow_forwardFor a red blood cell (erythrocyte) undergoing active glycolysis, briefly explain how increases in concentration of the following factors are likely to affect glycolytic flux. a. ATP b. AMP c. F-1,6-BP d. F-2,6-BP e. Citrate f. Glucose-6-phosphatearrow_forwardThe ∆G°’ for hydrolysis of phosphoenol pyruvate is -62.2 kJ/mol. The standard freeenergy of ATP hydrolysis is -30.5 kJ/mol. A. What is the standard free energy and K eq of the spontaneous reaction betweenADP/ATP and phosphoenol pyruvate. B. Repeat A for F-1,6-BP (∆G°’=-16.7 kJ/mol) and 1,3-BPG (∆G°’=-49.6 kJ/mol)hydrolysis. C. If the ATP and ADP concentrations are 8mM and 1mM respectively, what would bethe ratio of pyruvate/phosphoenolpyruvate at equilibrium?arrow_forward
- Answerarrow_forward13. Which one is the major organic product of the following sequence of reactions? A OH (CH3)2CHCH2COOH SOCI2 CH3OH 1. CH3MgBr 2. H₂O, H+ B C D OH E OHarrow_forward14. Which one is the major organic product of the following sequence of reactions? (CH3)2CH-COCI CH3OH 1. DIBALH, -78°C 1. PhCH2MgBr ? 2. H2O, HCI 2. H2O, HCI OH OMe A Ph B Ph OH Ph C OMe Ph D E OH .Pharrow_forward
- 6. Which one is the major organic product obtained from the following reaction? CO₂Me 1. LiAlH4 2. H₂O CH₂OH CH₂OCH3 5555 HO A B HO C HO D CH₂OH E ?arrow_forward1. (10 points) Pulverized coal pellets, which may be ° approximated as carbon spheres of radius r = 1 mm, are burned in a pure oxygen atmosphere at 1450 K and 1 atm. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C + O₂ →> CO₂. The reaction rate is first order and of the form No2 = k₁C₁₂(r), where k₁ = 0.1 m/s. Neglecting changes in r, determine the steady-state O₂ molar consumption rate in kmol/s. At 1450 K, the binary diffusion coefficient for O2 and CO2 is 1.71 x 10ª m²/s.arrow_forward2. (20 points) Consider combustion of hydrogen gas in a mixture of hydrogen and oxygen adjacent to the metal wall of a combustion chamber. Combustion occurs at constant temperature and pressure according to the chemical reaction 2H₂+ O₂→ 2H₂O. Measurements under steady-state conditions at 10 mm from the wall indicate that the molar concentrations of hydrogen, oxygen, and water vapor are 0.10, 0.10, and 0.20 kmol/m³, respectively. The generation rate of water vapor is 0.96x102 kmol/m³s throughout the region of interest. The binary diffusion coefficient for each of the species (H, O̟, and H₂O) in the remaining species is 0.6 X 10-5 m²/s. (a) Determine an expression for and make a qualitative plot of C as a function of distance from the wall. H2 (b) Determine the value of C2 at the wall. H2 (c) On the same coordinates used in part (a), sketch curves for the concentrations of oxygen and water vapor. This will require you to calculate Co, and C. 02 H20 (d) What is the molar flux of water…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage



Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning


Essentials of Pharmacology for Health Professions
Nursing
ISBN:9781305441620
Author:WOODROW
Publisher:Cengage

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license