
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
16th Edition
ISBN: 9781323406038
Author: McMurry
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6.1, Problem 6.4KCP
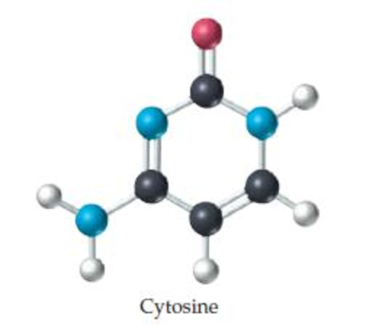
What is the molecular weight of cytosine, a component of DNA (deoxyribonucleic acid)? (black = C, blue = N, red = O, white = H.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. (10 points) Pulverized coal pellets, which may be
°
approximated as carbon spheres of radius r = 1 mm, are
burned in a pure oxygen atmosphere at 1450 K and 1 atm.
Oxygen is transferred to the particle surface by diffusion, where
it is consumed in the reaction C + O₂ →> CO₂. The reaction rate
is first order and of the form No2 = k₁C₁₂(r), where k₁ = 0.1 m/s.
Neglecting changes in r, determine the steady-state O₂ molar
consumption rate in kmol/s. At 1450 K, the binary diffusion
coefficient for O2 and CO2 is 1.71 x 10ª m²/s.
2. (20 points) Consider combustion of hydrogen gas in a mixture
of hydrogen and oxygen adjacent to the metal wall of a
combustion chamber. Combustion occurs at constant
temperature and pressure according to the chemical reaction
2H₂+ O₂→ 2H₂O. Measurements under steady-state conditions
at 10 mm from the wall indicate that the molar concentrations
of hydrogen, oxygen, and water vapor are 0.10, 0.10, and 0.20
kmol/m³, respectively. The generation rate of water vapor is
0.96x102 kmol/m³s throughout the region of interest. The binary
diffusion coefficient for each of the species (H, O̟, and H₂O) in
the remaining species is 0.6 X 10-5 m²/s.
(a) Determine an expression for and make a qualitative plot
of C as a function of distance from the wall.
H2
(b) Determine the value of C2 at the wall.
H2
(c) On the same coordinates used in part (a), sketch curves
for the concentrations of oxygen and water vapor. This will
require you to calculate Co, and C.
02
H20
(d) What is the molar flux of water…
4. (15 points) Consider a spherical organism of radius ro within
which respiration occurs at a uniform volumetric rate of That is,
oxygen (species A) consumption is governed by a first- order
reaction, homogeneous chemical reaction.
a. If a molar concentration of CA(ro) = CA,o is maintained at
the surface of the organism, obtain an expression for the
radial distribution of oxygen, CA(r), within the organism.
Hint: To simplify solution of the species diffusion equation,
invoke the transformation y = rCA.
b. Obtain an expression for the rate of oxygen consumption
within the organism.
c. Consider an organism of radius ro = 0.10 mm and a
diffusion coefficient of DAB = 108 m2/s. If CA, o = 5 x105
kmol/m3 and k1 20 s1, estimate the corresponding value
of the molar concentration at the center of the organism.
What is the rate of oxygen consumption by the organism?
Chapter 6 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
Ch. 6.1 - Calculate the molecular weight of the following...Ch. 6.1 - How many molecules of ascorbic acid (vitamin C,...Ch. 6.1 - What is the mass in grams of 5.0 1020 molecules...Ch. 6.1 - What is the molecular weight of cytosine, a...Ch. 6.2 - Prob. 6.5PCh. 6.2 - Prob. 6.6PCh. 6.2 - Prob. 6.7PCh. 6.3 - Prob. 6.8PCh. 6.3 - Prob. 6.9PCh. 6.4 - Hydrogen fluoride is one of the few substances...
Ch. 6.4 - The tungsten metal used for filaments in light...Ch. 6.5 - What is the theoretical yield of ethyl chloride in...Ch. 6.5 - The reaction of ethylene oxide with water to give...Ch. 6.5 - Prob. 6.14KCPCh. 6.5 - Dietary iron forms a 1:1 complex with hemoglobin...Ch. 6.5 - Prob. 6.2CIAPCh. 6.5 - Prob. 6.3CIAPCh. 6 - Methionine, an amino acid used by organisms to...Ch. 6 - Prob. 6.16UKCCh. 6 - Prob. 6.17UKCCh. 6 - Prob. 6.18UKCCh. 6 - Prob. 6.19UKCCh. 6 - Prob. 6.20APCh. 6 - What is the difference between molecular weight...Ch. 6 - Prob. 6.22APCh. 6 - Prob. 6.23APCh. 6 - How many calcium atoms are in 16.2 g of calcium?Ch. 6 - What is the mass in grams of 2.68 1022 atoms of...Ch. 6 - Calculate the molar mass of each of the following...Ch. 6 - Prob. 6.27APCh. 6 - Prob. 6.28APCh. 6 - Caffeine has the formula C8H10N4O2. If an average...Ch. 6 - How many moles of aspirin, C9H8O4, are in a 500 mg...Ch. 6 - What is the molar mass of diazepam (Valium),...Ch. 6 - Calculate the molar masses of the following...Ch. 6 - How many moles are present in a 4.50 g sample of...Ch. 6 - How many grams are present in a 0.075 mol sample...Ch. 6 - The principal component of many kidney stones is...Ch. 6 - Prob. 6.36APCh. 6 - Ethyl acetate reacts with H2 in the presence of a...Ch. 6 - Prob. 6.38APCh. 6 - Ammonia, NH3, is prepared for use as a fertilizer...Ch. 6 - Hydrazine, N2H4, a substance used as rocket fuel,...Ch. 6 - Prob. 6.41APCh. 6 - Magnesium metal burns in oxygen to form magnesium...Ch. 6 - Titanium metal is obtained from the mineral...Ch. 6 - Prob. 6.44APCh. 6 - Prob. 6.45APCh. 6 - Prob. 6.47APCh. 6 - Prob. 6.48APCh. 6 - Once made by heating wood in the absence of air,...Ch. 6 - In Problem 6.40, hydrazine reacted with oxygen...Ch. 6 - Dichloromethane, CH2Cl2, the solvent used to...Ch. 6 - Cisplatin [Pt(NH3)2Cl2], a compound used in cancer...Ch. 6 - Prob. 6.53APCh. 6 - Prob. 6.54APCh. 6 - Prob. 6.55CPCh. 6 - Prob. 6.56CPCh. 6 - Prob. 6.57CPCh. 6 - Prob. 6.58CPCh. 6 - Prob. 6.59CPCh. 6 - Prob. 6.60CPCh. 6 - Gaseous ammonia reacts with oxygen in the presence...Ch. 6 - Sodium hypochlorite, the primary component in...Ch. 6 - Barium sulfate is an insoluble ionic compound...Ch. 6 - The last step in the production of nitric acid is...Ch. 6 - Acetylsalicylic acid, the active ingredient in...Ch. 6 - Jewelry and tableware can be silver-plated by...Ch. 6 - Elemental phosphorus exists as molecules of P4. It...Ch. 6 - Lithium oxide is used aboard the International...Ch. 6 - Prob. 6.69CPCh. 6 - Prob. 6.70GPCh. 6 - Obtain a bottle of aspirin and identify the amount...Ch. 6 - Lovastatin, a drug used to lower serum...Ch. 6 - Pyrite, also known as fools gold, is used...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. (15 points) Living cells homogeneously distributed (immobilized) with an agarose gel require glucose to survive. An important aspect of the biochemical system design is the effective diffusion coefficient of glucose (A) into the cell- immobilized gel. Consider the experiment shows below where a slab of the cell-immobilized gel of 1.0cm thickness is placed within a well-mixed aqueous solution of glucose maintained at a concentration of 50 mmol/L. The glucose consumption within the cell-immobilized gel proceeds by a zero-order process given by R₁ = -0.05 mmol/(L min). The solubilities of glucose in both the water and the gel are the same; that is, the concentration of the glucose on the water side of the water-gel interface is equal to the concentration of the glucose on the gel side of the water gel interface. A syringe is mounted at the center of the gel carefully excises a tiny sample of the gel for glucose analysis. A Well mixed solution Constant concentration 50nmol/L Living…arrow_forwardTwo tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu Can you please help show the titration curve for both of these peptides and calculate the PI?arrow_forwardTwo tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu What is the structure of the PTH derivative produced during the last round of amino acid sequencing?arrow_forward
- What is the primary sequence of this undecapeptide? Also, if x-ray crystallography shows a highly stable hairpin turn within the polypeptide, what about the primary sequence explains this structural feature?arrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H H ⚫OH HO- -H H- -OH H- -OH CH2OH Ag*, NH4OH, H2O Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H₂O -OH H ⚫OH HO H HO- CH2OH Cu2+ Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. H、 H -OH H ⚫OH H -OH CH2OH Fehlings' solution ⑤ Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. HO C=0 H ⚫OH H ⚫OH HO- H HO H CH2OH Tollens' solution Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H-C=O HO H HO H H- ⚫OH HO H CH2OH HNO3, H2O Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. HO HO- HO H HO ∙H HO CH2OH NaBH4, CH3OH Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. Но сво HO H HO H H OH H -OH CH2OH H2 Pd Draw Fischer Projectionarrow_forwardDraw the Haworth projection for Gulose-ẞ-1,6-sorbose and answer the following questions. (Gulose will be in the pyranose form and Sorbose will be in the furanose form) a. Label the reducing and nonreducing ends of the disaccharide b. Label the glycosidic bond c. Circle the anomeric carbons and label them as hemiacetals or acetals. d. Can this disaccharide undergo mutarotation?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

DNA Use In Forensic Science; Author: DeBacco University;https://www.youtube.com/watch?v=2YIG3lUP-74;License: Standard YouTube License, CC-BY
Analysing forensic evidence | The Laboratory; Author: Wellcome Collection;https://www.youtube.com/watch?v=68Y-OamcTJ8;License: Standard YouTube License, CC-BY