
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 5Q
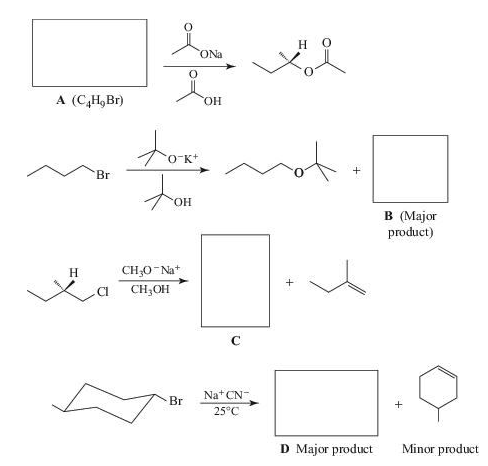
Supply the missing reactants, reagents, intermediates, or products.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
9. compore the Following two Venctions IN
termy Of Ronction Rate and explan in
detail the reasoning that led to your conclusion
+He p₁₂ 11-
ㅐ 15
.. +He
H #H
H
/
H
b. Compare
the Following too reactions 14
terms of reaction Rate and explain in detail
the reasoning that led to your conclusion
Н
d-C-
tłu
Na
+2446
е
-ll +2n
"H
a.
•Write all of the possible products
For the Following ronction
А
-----
H
-
H
H
+ H₂0 H+
Н
b. in Rite the complete reaction Mechaniszn
For the Formation of each product.
·C. Suggest what Reaction conditions could
Result in each product being the major
Product of the veaction:
a. Write the product For each of the
Following reactions
H
6-836-6
레
+H₂ N
A
H
A-C-C=C-C-CH + 2 Na +2 NH3 -
H H
b. Write the reaction Mechanism For.
reaction
each
Chapter 6 Solutions
Organic Chemistry
Ch. 6 - Prob. 1PPCh. 6 - Prob. 2PPCh. 6 - Prob. 3PPCh. 6 - Prob. 4PPCh. 6 - Prob. 5PPCh. 6 - PRACTICE PROBLEM 6.6
reactions that involve...Ch. 6 - PRACTICE PROBLEM 6.7 Rank the following...Ch. 6 - Prob. 8PPCh. 6 - Prob. 9PPCh. 6 - Prob. 10PP
Ch. 6 - Prob. 11PPCh. 6 - Prob. 12PPCh. 6 - Prob. 13PPCh. 6 - Prob. 14PPCh. 6 - Prob. 15PPCh. 6 - Prob. 16PPCh. 6 - Prob. 17PPCh. 6 - Prob. 18PPCh. 6 - Prob. 19PPCh. 6 - Prob. 20PCh. 6 - Prob. 21PCh. 6 - Which SN1 reaction of each pair would you expect...Ch. 6 - Prob. 23PCh. 6 - Prob. 24PCh. 6 - Listed below are several hypothetical nucleophilic...Ch. 6 - Prob. 28PCh. 6 - Write conformational structures for the...Ch. 6 - 6.28 Consider the reaction of with .
(a) Would...Ch. 6 - Prob. 33PCh. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - Prob. 36PCh. 6 - 1-Bromobicyclo[2, 2,1] heptane is extremely...Ch. 6 - When ethyl bromide reacts with potassium cyanide...Ch. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - When the alkyl bromides (listed here) were...Ch. 6 - Prob. 44PCh. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - 6.42 The reaction of chloroethane with water in...Ch. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Prob. 51PCh. 6 - Prob. 52PCh. 6 - 1-Bromo[2.2.1] bicycloheptane is unreactive toward...Ch. 6 - Open the computer molecular model tided...Ch. 6 - Prob. 56PCh. 6 - Consider the solvolysis reaction of (1S,...Ch. 6 - 2. Consider the following sequence of reactions,...Ch. 6 - Prob. 26PCh. 6 - Your task is to prepare isopropyl methyl ether by...Ch. 6 - Prob. 29PCh. 6 - 6.53 cis-4-Bromocyclohexanol racemic C6H10O...Ch. 6 - Prob. 31PCh. 6 - Explain the following observations: When...Ch. 6 - Prob. 38PCh. 6 - Prob. 1QCh. 6 - Prob. 2QCh. 6 - 6.3 A kinetic study yielded the following reaction...Ch. 6 - Prob. 4QCh. 6 - 6.5 Supply the missing reactants, reagents,...Ch. 6 - Which SN2 reaction will occur most rapidly....Ch. 6 - 6.7 Provide three-dimensional structures for the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
Chemistry (7th Edition)
3. Most battery-powered devices won’t work if you put the battery in backward. But for a device that you plug i...
College Physics: A Strategic Approach (3rd Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
29. For the reaction
determine the expression for the rate of the reaction in terms of the change in concentr...
Chemistry: Structure and Properties (2nd Edition)
14. How do the major histocompatibility complex class I and class II self-antigens function?
Principles of Anatomy and Physiology
Your bore cells, muscle cells, and skin cells look different because a. different kinds of genes are present in...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License