
Concept explainers
The following figure shows three 1.00-L bulbs connected by valves. Each bulb contains argon gas with amounts proportional to the number of circles pictorially represented in the chamber. All three bulbs are maintained at the same temperature. Unless stated otherwise, assume that the valves connecting the bulbs are closed and seal the gases in their respective chambers. Assume also that the volume between each bulb is negligible.
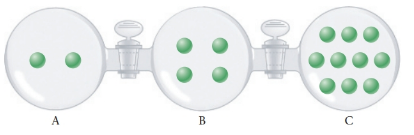
(a) Which bulb has the highest pressure?
(b) If the pressure in bulb A is 0.500 atm, what is the pressure in bulb C?
(c) If the pressure in bulb A is 0.500 atm, what is the total pressure?
(d) If the pressure in bulb A is 0.500 arm, and the valve between bulbs A and B is opened, redraw the figure shown above to accurately represent the gas atoms in all three bulbs. What is
(e) Follow the instructions of part (d) but now open only the valve between bulbs B and C.
(a)
Interpretation:
The bulb with the highest pressure needs to be identified based on the given description.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
Answer to Problem 86QAP
Bulb C has the highest pressure.
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in each of the bulbs can de deduced by substituting the values of n in bulbs A, B and C and volume, V = 1.0 L
Now, pressure (P) is directly proportional to the number of moles (n). Therefore, under constant temperature, bulb C will have the highest pressure.
(b)
Interpretation:
The pressure in bulb C needs to be deduced if the pressure in bulb A is 0.500 atm.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
Answer to Problem 86QAP
Pressure in Bulb C is 2.5 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in bulbs A and C can be deduced by substituting the given values of n, V and P under constant T
(c)
Interpretation:
The total pressure needs to be deduced if the pressure in bulb A is 0.500 atm.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure = 4.00 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in bulbs A and B can be deduced by substituting the given values of n, V and P under constant T
(d)
Interpretation:
The total pressure needs to be deduced after the valve between A and B is opened.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure after the valve between A and B is opened in 8.50 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
When the valve between A and B is opened the Ar gas will diffuse from the region of high pressure i.e. bulb B to A until equilibrium is established.
Now the total number of moles (atoms) of Ar = 2 + 4 = 6. The final pressure in each bulb will be due to 6 moles (atoms) of Ar
Step 1: Calculate the final pressure in bulb A after mixing:
The initial pressure in bulb A = Pi = 0.500 atm
Initial moles of Ar gas in A = ni = 2
Final moles in A = nf = 6
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 2: Calculate the final pressure in bulb B after mixing:
The initial pressure in bulb B = Pi = 1.50 atm
Initial moles of Ar gas in A = ni = 2
Final moles in A = nf = 6
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 3: Calculate the total pressure after mixing:
The total pressure after the valve between A and B is opened is higher than that when the valve is closed.
(d)
Interpretation:
The total pressure needs to be deduced after the valve between B and C is opened.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure after the valve between B and C is opened in 9.25 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
When the valve between B and C is opened the Ar gas will diffuse from the region of high pressure i.e. bulb C to B until equilibrium is established.
Now the total number of moles (atoms) of Ar = 4 + 10 = 14. The final pressure in each bulb will be due to 14 moles (atoms) of Ar
Step 1: Calculate the final pressure in bulb B after mixing:
The initial pressure in bulb B = Pi = 1.50 atm
Initial moles of Ar gas in B = ni = 4
Final moles in A = nf = 10
The final pressure in bulb B = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 2: Calculate the final pressure in bulb C after mixing:
The initial pressure in bulb C = Pi = 2.50 atm
Initial moles of Ar gas in C = ni = 10
Final moles in C = nf = 14
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 3: Calculate the total pressure after mixing:
The total pressure after the valve between B and C is opened is higher than that when the valve is closed.
Want to see more full solutions like this?
Chapter 5 Solutions
CHEMISTRY:PRIN.+REACTIONS-OWLV2 ACCESS
- Nonearrow_forwardA complete tensile test was performed on a magnesium specimen of 12 mm diameter and 30 mm length, until breaking. The specimen is assumed to maintain a constant volume. Calculate the approximate value of the actual stress at breaking. TABLE. The tensile force F and the length of the specimen are represented for each L until breaking. F/N L/mm 0 30,0000 30,0296 5000 10000 30,0592 15000 30,0888 20000 30,15 25000 30,51 26500 30,90 27000 31,50 26500 32,10 25000 32,79arrow_forwardNonearrow_forward
- Differentiate between plastic deformation, elastic deformation, viscoelastic deformation and viscoplastic deformation.arrow_forward1.57 Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid.arrow_forwardFor the two questions below, draw the mechanism and form the major product.arrow_forward
- Indicate similarities and differences between natural, exchanged and pillared clays.arrow_forwardShow work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forward
- This thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward7. Draw the mechanism to describe the following transformation: Note: This is a base catalyzed reaction. So, the last steps must make [OH]- OH [OH]¯ OH Heat Oarrow_forwardShow work with explanation...don't give Ai generated solutionarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





