
Concept explainers
(a)
Interpretation:
The tank with the highest total pressure needs to be determined.
Concept introduction:
According to the
When at two different conditions gases are placed, then to determine the changed variable combined gas law is used. Below is the formula of combined gas law:
Here
- P1 and P2 are the pressure of gases
- V1 and V2 and volume of gases
- n1 and n2 number of moles
- T1 and T2 are the temperature of gases
Moles are known as the ratio of mass and molar mass. Below is the formula:
Here, MM is molar mass and m is the mass.
The kinetic model of gases is accounted for ideal gas behavior. The formula of average translational energy of gas is as below:
Here,
Et = average translational energy of gas
T = temperature in Kelvin
R = Universal gas constant
NA =
Effusion is known as the leakage of gas molecules from high to low pressure region via a pinhole. For any two gas molecules the formula to determine the time needed for effusion is as below:
Here u1 and u2 is the rate of effusion for gas1 and gas 2. MM1 and MM2 is the molar mass for gas1 and gas 2.
Answer to Problem 87QAP
Tank Y
Explanation of Solution
The given tanks are as follows:
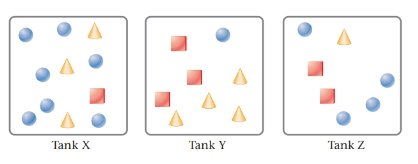
Here, circles represent
The highest total pressure is in the tank Y. Because, tank Y has the maximum amount of gases mixture.
(b)
Interpretation:
The tank containing highest SO2 pressure needs to be determined.
Concept introduction:
According to the ideal
When at two different conditions gases are placed, then to determine the changed variable combined gas law is used. Below is the formula of combined gas law:
Here
- P1 and P2 are the pressure of gases
- V1 and V2 and volume of gases
- n1 and n2 number of moles
- T1 and T2 are the temperature of gases
Moles are known as the ratio of mass and molar mass. Below is the formula:
Here, MM is molar mass and m is the mass.
The kinetic model of gases is accounted for ideal gas behavior. The formula of average translational energy of gas is as below:
Here,
Et = average translational energy of gas
T = temperature in Kelvin
R = Universal gas constant
NA = Avogadro number
Effusion is known as the leakage of gas molecules from high to low pressure region via a pinhole. For any two gas molecules the formula to determine the time needed for effusion is as below:
Here, u1 and u2 is the rate of effusion for gas1 and gas 2. MM1 and MM2 is the molar mass for gas1 and gas 2.
Answer to Problem 87QAP
Tank Y
Explanation of Solution
The given tanks are as follows:
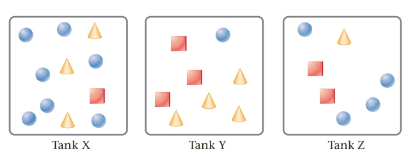
Here, circles represent
Tank Y contains highest partial pressure of SO2 . This is because, Tank Y has the maximum moles of SO2 due to which it will have the maximum partial pressure.
(c)
Interpretation:
The tank with same mass of all the three gases needs to be determined.
Concept introduction:
According to the ideal gas law volume i.e. V, pressure i.e. P, number of moles i.e. m, temperature i.e. t and universal gas constant i.e. R are interrelated as below:
When at two different conditions gases are placed, then to determine the changed variable combined gas law is used. Below is the formula of combined gas law:
Here
- P1 and P2 are the pressure of gases
- V1 and V2 and volume of gases
- n1 and n2 number of moles
- T1 and T2 are the temperature of gases
Moles are known as the ratio of mass and molar mass. Below is the formula:
Here, MM is molar mass and m is the mass.
The kinetic model of gases is accounted for ideal gas behavior. The formula of average translational energy of gas is as below:
Here,
Et = average translational energy of gas
T = temperature in Kelvin
R = Universal gas constant
NA = Avogadro number
Effusion is known as the leakage of gas molecules from high to low pressure region via a pinhole. For any two gas molecules the formula to determine the time needed for effusion is as below:
Here, u1 and u2 is the rate of effusion for gas1 and gas 2. MM1 and MM2 is the molar mass for gas1 and gas 2.
Answer to Problem 87QAP
Tank Z
Explanation of Solution
The given tanks are as follows:
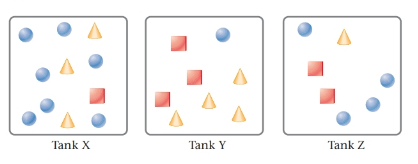
Here, circles represent
Tank Z contain the mass of all three gases same.
(d)
Interpretation:
The tank with the heaviest content needs to be determined.
Concept introduction:
According to the ideal gas law volume i.e. V, pressure i.e. P, number of moles i.e. m, temperature i.e. t and universal gas constant i.e. R are interrelated as below:
When at two different conditions gases are placed, then to determine the changed variable combined gas law is used. Below is the formula of combined gas law:
Here
- P1 and P2 are the pressure of gases
- V1 and V2 and volume of gases
- n1 and n2 number of moles
- T1 and T2 are the temperature of gases
Moles are known as the ratio of mass and molar mass. Below is the formula:
Here, MM is molar mass and m is the mass.
The kinetic model of gases is accounted for ideal gas behavior. The formula of average translational energy of gas is as below:
Here,
Et = average translational energy of gas
T = temperature in Kelvin
R = Universal gas constant
NA = Avogadro number
Effusion is known as the leakage of gas molecules from high to low pressure region via a pinhole. For any two gas molecules the formula to determine the time needed for effusion is as below:
Here u1 and u2 is the rate of effusion for gas1 and gas 2. MM1 and MM2 is the molar mass for gas1 and gas 2.
Answer to Problem 87QAP
Tank Y
Explanation of Solution
The given tanks are as follows:
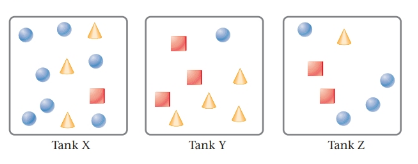
Here, circles represent
Tank Y contains the heaviest content. This is due to the number of moles of SO2 is highest in tank Y.
Want to see more full solutions like this?
Chapter 5 Solutions
CHEMISTRY:PRIN.+REACTIONS-OWLV2 ACCESS
- Draw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction 'O 10 + x 也 HO + 义 Click and drag to start drawing a structure.arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H- :0: C=N: b Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0° b=0 Xarrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + This transformation can't be done in one step. T iarrow_forward
- Determine the structures of the missing organic molecules in the following reaction: H+ O OH H+ + H₂O ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. X § ©arrow_forwardTable 1.1 Stock Standard Solutions Preparation. The amounts shown should be dissolved in 100 mL. Millipore water. Calculate the corresponding anion concentrations based on the actual weights of the reagents. Anion Amount of reagent (g) Anion Concentration (mg/L) 0.1649 Reagent Chloride NaCl Fluoride NaF 0.2210 Bromide NaBr 0.1288 Nitrate NaNO3 0.1371 Nitrite NaNO2 0.1500 Phosphate KH2PO4 0.1433 Sulfate K2SO4 0.1814arrow_forwardDraw the structure of the pound in the provided CO as a 300-1200 37(2), 11 ( 110, and 2.5 (20arrow_forward
- Please help me with # 4 and 5. Thanks in advance!arrow_forwardA small artisanal cheesemaker is testing the acidity of their milk before it coagulates. During fermentation, bacteria produce lactic acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and develop flavor. The cheesemaker has measured that the developing mixture contains lactic acid at an initial concentration of 0.025 M. Your task is to calculate the pH of this mixture and determine whether it meets the required acidity for proper cheese development. To achieve the best flavor, texture and reduce/control microbial growth, the pH range needs to be between pH 4.6 and 5.0. Assumptions: Lactic acid is a monoprotic acid H H :0:0: H-C-C H :0: O-H Figure 1: Lewis Structure for Lactic Acid For simplicity, you can use the generic formula HA to represent the acid You can assume lactic acid dissociation is in water as milk is mostly water. Temperature is 25°C 1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to include state symbols.…arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: :0 H. 0:0 :0: :6: S: :0: Select to Edit Arrows ::0 Select to Edit Arrows H :0: H :CI: Rotation Select to Edit Arrows H. < :0: :0: :0: S:arrow_forward
- 3:48 PM Fri Apr 4 K Problem 4 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Mg. :0: Select to Add Arrows :0: :Br: Mg :0: :0: Select to Add Arrows Mg. Br: :0: 0:0- Br -190 H 0:0 Select to Add Arrows Select to Add Arrows neutralizing workup H CH3arrow_forwardIarrow_forwardDraw the Markovnikov product of the hydrobromination of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. + Explanation Check 1 X E 4 1 1 1 1 1 HBr Click and drag to start drawing a structure. 80 LE #3 @ 2 $4 0 I அ2 % 85 F * K M ? BH 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center & 6 27 FG F10 8 9 R T Y U D F G H P J K L Z X C V B N M Q W A S H option command H command optiarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





