
Concept explainers
(a)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule does not have any chiral center, and it is not a meso compound.
Explanation of Solution
The structure of the given molecule is
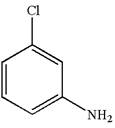
A chiral center must be an
The given molecule is determined as not a meso compound as it has no chiral center.
(b)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has one chiral center marked with
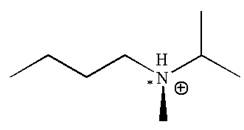
Explanation of Solution
The structure of the given molecule is
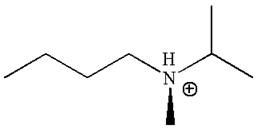
In this molecule, the nitrogen atom is a chiral center bonded to four different groups
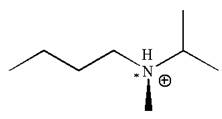
As this molecule has only one chiral center, it cannot possess any symmetry, and hence, it is not a meso compound.
The chiral center in the given molecule is identified, and it is determined that the molecule is not a meso compound.
(c)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has one chiral center marked with
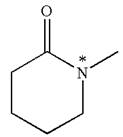
Explanation of Solution
The structure of the given molecule is
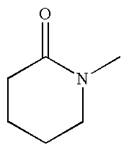
The molecule consists of a ring made up of five carbon atoms and one nitrogen atom. The nitrogen atom is bonded to three different groups having the pyramidal shape and a non-bonded electron pair pointing to the unoccupied tetrahedral corner. This makes the nitrogen a chiral center.
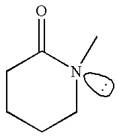
As this molecule has only one chiral center, it cannot possess any symmetry, and hence, it is not a meso compound. The chiral center is marked as
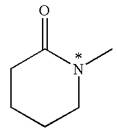
The chiral center in the given molecule is identified, and it is determined that the molecule is not a meso compound.
(d)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
The molecule with at least one chiral center having no plane of symmetry is called a chiral molecule. A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has no chiral center, and it is not a meso compound.
Explanation of Solution
The structure of the given molecule is
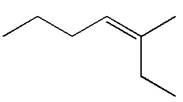
A chiral center must be an
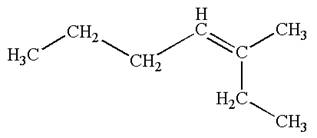
Therefore, these carbon atoms are also not chiral centers. As there are no chiral centers, the molecule is not a meso compound.
The given molecule is determined as not a meso compound as it has no chiral center.
(e)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The molecule has two chiral centers marked with
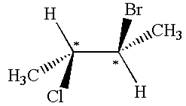
It is not a meso compound.
Explanation of Solution
The structure of the given molecule is
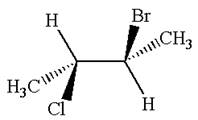
The given molecule possesses two chiral carbons. One carbon is bonded to four different groups,
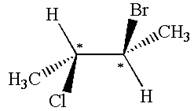
The molecule does not have symmetry plane; hence, it is not a meso compound.
The chiral centers in the given molecule are identified, and it is determined that the molecule is not a meso compound.
(f)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
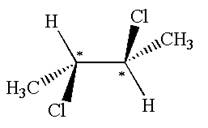
The molecule has two chiral centers marked with
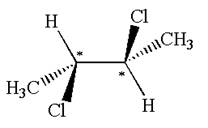
Explanation of Solution
The structure of the given molecule is
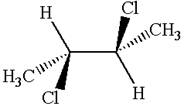
The given molecule possesses two chiral carbons bonded to four different groups,
The molecule has no plane of symmetry, and hence, it is not a meso compound.
The chiral centers in the given molecule are identified, and it is determined that the molecule is not a meso compound.
(g)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
The molecule with at least one chiral center having no plane of symmetry is called a chiral molecule. A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The molecule has two chiral centers marked with
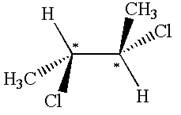
Explanation of Solution
The structure of the given molecule is
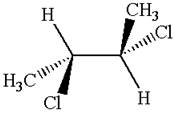
The given molecule possesses two chiral carbons bonded to four different groups,
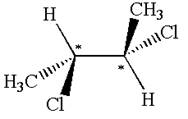
The molecule has no symmetry plane, and hence, it is not a meso compound.
The chiral centers in the given molecule are identified, and it is determined that the molecule is not a meso compound.
(h)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
The molecule with at least one chiral center having no plane of symmetry is called chiral molecule. A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has one chiral center marked with
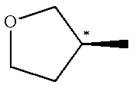
Explanation of Solution
The structure of the given molecule is
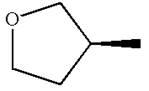
The molecule consists of a ring made up of four carbon atoms and one oxygen atom with a substituted methyl group. The carbon having the methyl substituent is a chiral center as it has four different groups bonded.
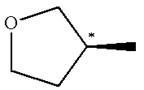
The molecule does not possess any symmetry plane; hence, it is a chiral molecule.
The chiral center in the given molecule is identified, and it is determined that the molecule is not a meso compound.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- What is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardStuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward
- 个 Stuc X ctclix ALE X A ALE × A ALE X Lab x (195 × Nut x M Inbx EF 目 → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpz Chapter 12 HW = Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited Part: 1/2 Part 2 of 2 Give the IUPAC name. Check 3 50°F Clear ©2025 McGraw Hill L Q Search webp a عالياكarrow_forward个 Stuck x ctc xALE X A ALE × A ALE X Lab x (19: x - G www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-1Q1g8NUi-mObka ZLx2twjEhK7mVG6PUUIO06 Chapter 12 HW 三 Question 26 of 39 (4 points) 1 Question Attempt: 1 of Unlimited Answer the following questions about the given alkane. Part: 0 / 2 Part 1 of 2 Give the IUPAC name. Skip Part 2 53°F Clear Check × Q Search hp hp 02arrow_forwardCalculate the equilibrium constant at 25.0 oC for the following equation. Cd(s) + Sn+2(aq) ↔Cd+2(aq) + Sn(s) Group of answer choices 3.11x104 1.95x1018 9.66x108 1.40x109arrow_forward
- What is the pH at the cathode for the following cell written in line notation at 25.0 oC with a Ecell = -0.2749 V? Ni(s)|Ni+2(aq, 1.00 M)||H+1(aq, ?M)|H2(g, 1.00 atm)|Pt(s)arrow_forwardCalculate Ecell for a hydrogen fuel cell at 95.0 oC using the following half-reactions with PH2 = 25.0 atm and PO2 = 25.0 atm. O2(g) + 4H+1(aq) + 4e-1 → 2H2O(l) Eo = 1.229 V 2H2(g) → 4H+1(aq) + 4e-1 Eo = 0.00 Varrow_forwardCalculate Ecell at 25.0 oC using the following half-reactions with [Ag+1] = 0.0100 M and [Sn+2] = 0.0200 M. Ag+1(aq) + 1e-1 Ag(s) Sn+2(aq) + 2e-1 Sn(s)arrow_forward
- Done 18:19 www-awu.aleks.com Chapter 12 HW Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited .. LTE סוי 9 ✓ 20 ✓ 21 × 22 23 24 25 26 27 28 29 30 Answer the following questions about the given alkane. Part: 0 / 2 Part 1 of 2 Classify each carbon atom as a 1º, 2º, 3º, or 4°. Highlight in red any 1° carbons, highlight in blue any 2° carbons. highlight in green any 3° carbons, and leave any 4° carbons unhighlighted. Skip Part Check Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility ☑ คarrow_forward< Done 19:22 www-awu.aleks.com Chapter 12 HW Question 4 of 39 (2 points) | Question Attempt: 5 of Unlimited : .. LTE סוי 1 ✓ 2 ✓ 3 = 4 ✓ 5 ✓ 6 ✓ 7 ✓ 8 ✓ 9 = 10 11 ✓ 12 Consider the molecule (CH3)2CHCH2CHCн for the following questions. Part 1 of 2 Which of the following molecules is/are constitutional isomer(s) to (CH3)2CHCH2CH2CH3? Check all that apply. Part 2 of 2 (CH3),C(CH2)2CH3 CH3 H,C-CH-CH-CH, CH 3 None of the above. ☑ Which of the following molecules is/are identical molecules to (CH3)2CHCH2CH2CH₁₂? Check all that apply. CH3 H,C-CH-CH₂-CH2-CH, CH3(CH2)2CH(CH3)2 CH2-CH2-CH3 HỌC-CH=CH, 乂 ☑ а None of the above Check Save For Later Submit Assignment © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward18:11 LTE ا... US$50 off hotels is waiting for you Book now, hotels in Nashville are going fast QUTSLIVII 25 61 69 points) | QuestIVIT ALLēm... now Give the IUPAC name for each compound. Part 1 of 3 Part 2 of 3 X ☑ Х Check Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. TOMS CT US ...vacy Center | Accessibilityarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





