
Concept explainers
(a)
Interpretation:
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
The Fischer projection of the given molecule is
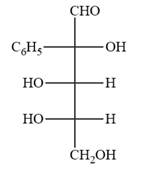
Explanation of Solution
The given molecule is
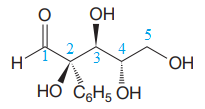
The given molecule consists of a five carbon chain, each of which is numbered. C2, C3, and C4 are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:
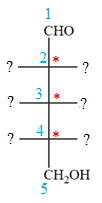
The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C2 and C3 carbon atoms lie on the same side of the plane and are shown by a wedge bond. Thus, these two groups must lie on opposite sides in the Fischer projection, as shown below:
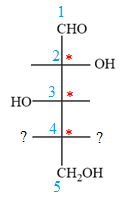
The OH groups on C3 and C4 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on the same side in the Fischer projection, as shown below:
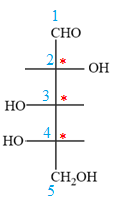
The OH groups on C2 and C4 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on the opposite side in the Fischer projection.
Thus, the OH groups on C3 and C4 carbon atoms must lie on the same side of the vertical chain, and OH group on C2 must lie on the opposite side. Then fill in the remaining groups on C2, C3, and C4 carbon atoms to complete the structure as below:
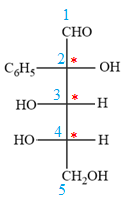
Thus, the structure above is the correct conversion from a given zig-zag conformation to a Fischer projection.
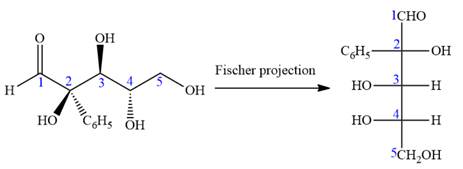
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Interpretation:
(b)
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
Answer to Problem 5.52P
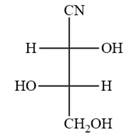
The Fischer projection of the given molecule is
Explanation of Solution
The given molecule is
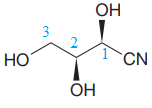
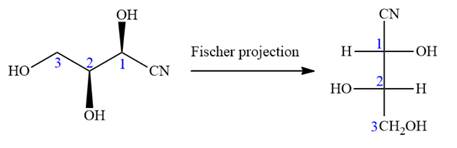
The given molecule consists of a three carbon chain, each of which is numbered. C1 and C2 carbon atoms are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:
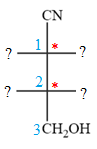
The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C1 and C2 carbon atoms lie on the same side of the plane and are shown by a wedge bond. Thus, these two groups must lie on opposite side in the Fischer projection, as shown below:
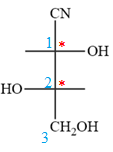
Then fill in the remaining groups on C1 and C2 carbon atoms to complete the structure as below:
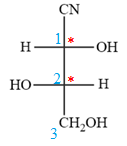
Thus, the structure above is the correct conversion from a given zig-zag conformation to a Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Interpretation:
(c)
Fischer projection of the given molecule is to be drawn.
Concept introduction:
A molecule containing a chain of carbon atoms is frequently represented in its zigzag conformation. If it contains multiple asymmetric centers, it is more convenient to draw its Fisher projection. Fischer projections are generally drawn with the longest carbon chain vertical. The two groups attached to each carbon except the first and last are shown on horizontal bonds. The carbon atoms in the middle of the chain are represented by the intersections of vertical and horizontal lines. The vertical lines represent bonds that are oriented away from the viewer while the horizontal bonds are oriented toward the viewer. The most oxidized group must be present at the top of the vertical line.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the same side of the plane and are shown by wedge bonds, then those groups will be on the opposite sides of the vertical chain in the Fischer projection.
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the same side of the vertical chain.
In the zig-zag conformation, if two groups on alternate carbon atoms are on opposite sides of the plane and are shown by a wedge and a dash bond, then, in the Fischer projection, those groups will be on the opposite sides of the vertical chain.
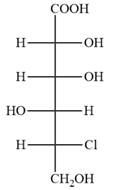
Answer to Problem 5.52P
The Fischer projection of the given molecule is
Explanation of Solution
The given molecule is
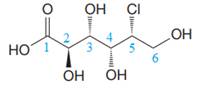
The given molecule consists of a six carbon chain, each of which is numbered. C2, C3, C4, and C5 carbon atoms are the asymmetric carbon atoms. We begin to draw the Fischer projection with the framework shown below:

The asymmetric centers are denoted by asterisks. Two of the bonds on each asymmetric center have been left with question marks because two substituents must still be added to each so that the stereochemical configuration at those carbon atoms in the Fischer projection matches with what was given in the dash-wedge notation.
Notice that in the zig-zag conformation, the OH groups on C2 and C3 carbon atoms lie on the opposite side of the plane and are shown by a wedge and a dash bond. Thus, these two groups must lie on same side in the Fischer projection, as shown below:
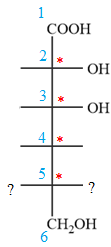
Notice that in the zig-zag conformation, the OH groups on C3 and C4 carbon atoms lie on the same side of the plane and are shown by dash bonds. Thus, these two groups must lie on the opposite sides of the vertical chain in the Fischer projection, as shown below:
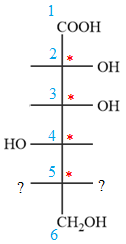
In the zig-zag conformation, the OH group and chlorine atom on C4 and C5 carbon atoms lie on the same side of the plane and are shown by dash bonds. Thus, these two groups must lie on the opposite sides of the vertical chain in the Fischer projection, as shown below:
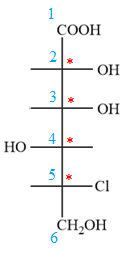
Then place the remaining groups on C1 and C2 carbon atoms to complete the structure as below:
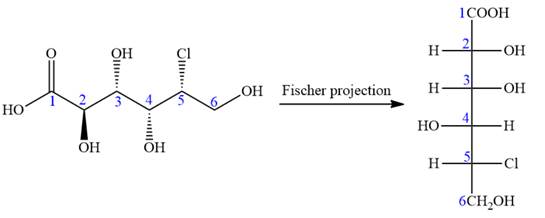
In the zig-zag conformation, if two groups on adjacent carbon atoms are on the opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection. In the zig-zag conformation, if two groups on adjacent carbon atoms are on opposite sides of the plane and are shown by a wedge and dash bond, then those groups will be on the same side of the vertical chain in the Fischer projection.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



