
(1)
The phases present in chemical composition.
(1)
Explanation of Solution
Introduction:
A phaseis a homogeneous portion of a material that has uniform physical and chemical characteristics. A phase transformation occurs when a material changes from one phase to another. Phase transformations enter into many material processes, such as when parts are soldered or welded, or when metal is melted and cast into a shape that is then cooled to form a solid part.
(a)
The composition of
(b)
The composition of
(c)
The composition of
(d)
The composition of
(e)
The composition of
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Phase |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 |
Table (1)
Conclusion:
Therefore, the phases present in chemical composition is shown in Table (1).
(2)
The degree of freedom in the material from Gibbs phase rule.
(2)
Answer to Problem 5.13P
The degree of freedom is shown in Table (2) .
Explanation of Solution
Formula Used:
Write the expression for the Gibbs phase rule as:
Here,
Calculation:
(a)
Substitute
(b)
Substitute
(c)
Substitute
(d)
Substitute
(e)
Substitute
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Degree of freedom |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 |
Table (2)
Conclusion:
Therefore, the degree of freedom is shown in Table (2) .
(3)
The chemical composition of phase.
(3)
Explanation of Solution
Introduction:
Many two-component materials have two phases in equilibrium that have different chemical compositions. Low-carbon steel has the two components iron and carbon, and at room temperature it has two phases. The first phase is
iron carbide (Fe3C).
(a)
The chemical composition present at the temperature of
(b)
The chemical composition present at the temperature of
Thus,
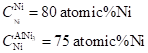
(c)
The chemical composition present at the temperature of
Thus,
(d)
The chemical composition present at the temperature of
Thus,
(e)
The chemical composition present at the temperature of
Thus,
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Chemical composition |
| 1 | |||
| 2 | 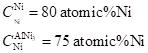 | ||
| 3 |
| ||
| 4 |
| ||
| 5 |
|
Table (3)
Conclusion:
Thus, the chemical composition of phase is shown in Table (3).
(4)
The atomic fraction of each phase.
(4)
Answer to Problem 5.13P
The atomic fraction for each phase is shown in Table (4) .
Explanation of Solution
Formula Used:
For
Write the expression for the atomic fraction of the
Here,
For
Write the expression for the atomic fraction of the
For
Write the expression for the atomic fraction of the
Here,
For
Write the expression for the atomic fraction of the
Calculation:
(a)
The atomic fraction of the Ni phase at the temperature of
(b)
Substitute
Substitute
(c)
Substitute
Substitute
(d)
The Peritectic reaction occurs at the temperature of
(e)
The atomic fraction of
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Atomic fraction |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | Not possible | ||
| 5 |
Table (4)
Conclusion:
Thus, the atomic fraction for each phase is shown in Table (4) .
Want to see more full solutions like this?
Chapter 5 Solutions
Materials Science And Engineering Properties
- Determine the stiffness matrices of elements 2, 3 and 4 in the global co-ordinate system. Assume A=0.0015m2 and E=200GPa, indicate the degrees of freedom in all stiffness matricies.arrow_forwardA short plain concrete column with cross-section dimensions of 12 in x 12 in is to be constructed. If the compressive strength of the concrete (f’c) is 5000 psi, what is the maximum load that can be safely applied to the column? - 600 k - 950 k - 720 k - 347 karrow_forwardThe borrow pit has 2000 cyds of suitable fill. The fill required for the project is 1900 cyds. The swell factor is 10% and the shrinkage factor is 15%. How much more borrow do we need? Or is there extra? - 13 yards extra - 13 yards short - 200 yards extra - 161 yards shortarrow_forward
- The job site has a primary vertical control point with a reference benchmark of 100 ft. An instrument is set up with an HI of 5.42 ft above the BM. A grade stake is set at an elevation of 96 ft. What is the height reading on the rod at the grade stake? - 9.42 ft - 4.00 ft - 1.42 ft - 5.42 ftarrow_forwardAssume you have a simple beam 16 ft long supported on each end by R1 and R2. There is a concentrated load of 900 lb that is 4 ft from R2. Reaction R1 is pinned 12 ft from the load. Reaction R1 is 225 lb and R2 is 675 lb. What is the maximum bending moment in pounds per foot? - 3,600 - 1,800 - 2,700- 900arrow_forwardA wall form is four-feet high, ten-feet long and ten-feet wide. It is full of fluid concrete. What is the pressure at the bottom of the form? - 86,400 psi - 60,000 psf - 600 psf - 60,000 psiarrow_forward
- he sides of a building are 300 feet long and 200 feet wide. What is the diagonal distance between the opposite corners? - 300 feet - 306 feet - 360.60 feet - 306.6 feetarrow_forwardThe excavation for a square basement requires a 1:2 H:V slope cut back. The external dimensions of the footings are 30 x 30 feet. The excavation is 8 feet deep and you need to add 2 feet on each side to install the footing forms. What is the volume of cubic yards of excavation? - 486.7cy - 13140cy - 858.8cy - 429.4cyarrow_forwardThe Modulus of Elasticity is 30,000,000 for mild carbon steel. In the plastic state, the unit stress is 25000 psi. What is the strain? - 1.45 - 145.0 - 0.00083 - 1450.0arrow_forward
- The building is twenty-five feet by twenty five feet. The side line of the building and two corners along the line have been staked. What is the diagonal distance and the 90 degree distance to the other corners? - 35.36 feet and 35.36 feet - 25.00 feet and 35.36 feet - 35.36 feet and 25 feet - 25.00 feet and 25.00 feetarrow_forwardNEED HELP THANK YOU IN ADVACEarrow_forwardNEED HELP THANK YOU IN ADVANCEarrow_forward
 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
