
Thermodynamics: An Engineering Approach
8th Edition
ISBN: 9780073398174
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.5, Problem 21P
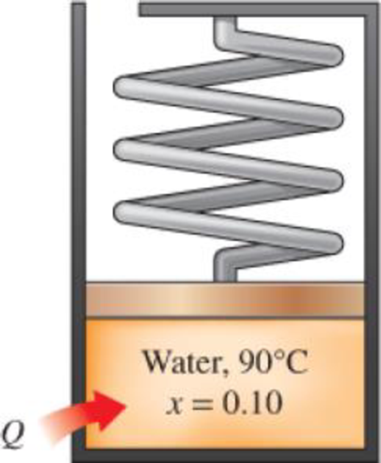
1 kg of water that is initially at 90°C with a quality of 10 percent occupies a spring-loaded piston–cylinder device, such as that in Fig. P4–22. This device is now heated until the pressure rises to 800 kPa and the temperature is 250°C. Determine the total work produced during this process, in kJ.
FIGURE P4–22
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The heated rod from Problem 3 is subject to a volumetric heating
h(x) = h0
x
L in units of [Wm−3], as shown in the figure below. Under the
heat supply the temperature of the rod changes along x with the
temperature function T (x). The temperature T (x) is governed by the
d
following equations:
−
dx (q(x)) + h(x) = 0 PDE
q(x) =−k dT
dx Fourier’s law of heat conduction (4)
where q(x) is the heat flux through the rod and k is the (constant)
thermal conductivity. Both ends of the bar are in contact with a heat
reservoir at zero temperature.
Determine:
1. Appropriate BCs for this physical problem.
2. The temperature function T (x).
3. The heat flux function q(x).
Side Note: Please see that both ends of bar are in contact with a heat reservoir at zero temperature so the boundary condition at the right cannot be du/dx=0 because its not thermally insulated. Thank you
The elastic bar from Problem 1 spins with angular velocity ω about an axis, as shown in the figure below. The radial acceleration at a generic point x along the bar is a(x) = ω2x. Under this radial acceleration, the bar stretches along x with displacement function u(x). The displacement d u(x) is governed by the following equations: dx (σ(x)) + ρa(x) = 0 PDE σ(x) = E du dx Hooke’s law (2) where σ(x) is the axial stress in the rod, ρ is the mass density, and E is the (constant) Young’s modulus. The bar is pinned on the rotation axis at x = 0 and it is also pinned at x = L. Determine: 1. Appropriate BCs for this physical problem. 2. The displacement function u(x). 3. The stress function σ(x). SIDE QUESTION: I saw a tutor solve it before but I didn't understand why the tutor did not divide E under the second term (c1x) before finding u(x). The tutor only divided E under first term. please explain and thank you
calculate the total power required to go 80 mph in a VW Type 2 Samba Bus weighing 2310 lbs. with a Cd of 0.35 and a frontal area of 30ft^2. Consider the coefficient of rolling resistance to be 0.018. What is the increase in power required to go the same speed if the weight is increased by 2205 pounds (the rated carrying capacity of the vehicle). If the rated power for the vehicle is 49 bhp, will the van be able to reach 80 mph at full carrying capacity?
Chapter 4 Solutions
Thermodynamics: An Engineering Approach
Ch. 4.5 - An ideal gas at a given state expands to a fixed...Ch. 4.5 - Nitrogen at an initial state of 300 K, 150 kPa,...Ch. 4.5 - 4–3 The volume of 1 kg of helium in a...Ch. 4.5 - 4–4E Calculate the total work, in Btu, for process...Ch. 4.5 - 4–5 A piston–cylinder device initially contains...Ch. 4.5 - A pistoncylinder device with a set of stops...Ch. 4.5 - 4–7 A piston–cylinder device initially contains...Ch. 4.5 - 4–8 A mass of 5 kg of saturated water vapor at 300...Ch. 4.5 - 1 m3 of saturated liquid water at 200C is expanded...Ch. 4.5 - A gas is compressed from an initial volume of 0.42...
Ch. 4.5 - A mass of 1.5 kg of air at 120 kPa and 24C is...Ch. 4.5 - During some actual expansion and compression...Ch. 4.5 - 4–14 A frictionless piston–cylinder device...Ch. 4.5 - Prob. 15PCh. 4.5 - During an expansion process, the pressure of a gas...Ch. 4.5 - A pistoncylinder device initially contains 0.4 kg...Ch. 4.5 - 4–19E Hydrogen is contained in a piston–cylinder...Ch. 4.5 - A pistoncylinder device contains 0.15 kg of air...Ch. 4.5 - 1 kg of water that is initially at 90C with a...Ch. 4.5 - Prob. 22PCh. 4.5 - An ideal gas undergoes two processes in a...Ch. 4.5 - A pistoncylinder device contains 50 kg of water at...Ch. 4.5 - Prob. 26PCh. 4.5 - 4–27E A closed system undergoes a process in which...Ch. 4.5 - A rigid container equipped with a stirring device...Ch. 4.5 - A 0.5-m3rigid tank contains refrigerant-134a...Ch. 4.5 - A 20-ft3 rigid tank initially contains saturated...Ch. 4.5 - Prob. 31PCh. 4.5 - Prob. 32PCh. 4.5 - Prob. 33PCh. 4.5 - An insulated pistoncylinder device contains 5 L of...Ch. 4.5 -
4–35 A piston–cylinder device initially...Ch. 4.5 - Prob. 37PCh. 4.5 - A 40-L electrical radiator containing heating oil...Ch. 4.5 - Steam at 75 kPa and 8 percent quality is contained...Ch. 4.5 - Prob. 40PCh. 4.5 - An insulated tank is divided into two parts by a...Ch. 4.5 - Is the relation u = mcv,avgT restricted to...Ch. 4.5 - Is the relation h = mcp,avgT restricted to...Ch. 4.5 - Is the energy required to heat air from 295 to 305...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - Prob. 49PCh. 4.5 - What is the change in the enthalpy, in kJ/kg, of...Ch. 4.5 - Prob. 51PCh. 4.5 - Prob. 52PCh. 4.5 - Prob. 53PCh. 4.5 - Determine the internal energy change u of...Ch. 4.5 - Prob. 55PCh. 4.5 - Prob. 56PCh. 4.5 - Is it possible to compress an ideal gas...Ch. 4.5 - A 3-m3 rigid tank contains hydrogen at 250 kPa and...Ch. 4.5 - A 10-ft3 tank contains oxygen initially at 14.7...Ch. 4.5 - 4–60E A rigid tank contains 10 Ibm of air at 30...Ch. 4.5 - 4–61E Nitrogen gas to 20 psia and 100°F initially...Ch. 4.5 - An insulated rigid tank is divided into two equal...Ch. 4.5 - 4–63 A 4-m × 5-m × 6-m room is to be heated by a...Ch. 4.5 - 4-64 A student living in a 3-m × 4-m × 4-m...Ch. 4.5 - A 4-m 5-m 7-m room is heated by the radiator of...Ch. 4.5 - 4–66 Argon is compressed in a polytropic process...Ch. 4.5 - An insulated pistoncylinder device contains 100 L...Ch. 4.5 - 4–68 A spring-loaded piston-cylinder device...Ch. 4.5 - An ideal gas contained in a pistoncylinder device...Ch. 4.5 - Air is contained in a variable-load pistoncylinder...Ch. 4.5 - Prob. 71PCh. 4.5 - Prob. 72PCh. 4.5 - Prob. 74PCh. 4.5 - Prob. 75PCh. 4.5 - Prob. 76PCh. 4.5 - 4–77 Air is contained in a piston-cylinder device...Ch. 4.5 - A pistoncylinder device contains 4 kg of argon at...Ch. 4.5 - The state of liquid water is changed from 50 psia...Ch. 4.5 - During a picnic on a hot summer day, all the cold...Ch. 4.5 - Consider a 1000-W iron whose base plate is made of...Ch. 4.5 - Stainless steel ball bearings ( = 8085 kg/m3 and...Ch. 4.5 - In a production facility, 1.6-in-thick 2-ft 2-ft...Ch. 4.5 - Prob. 84PCh. 4.5 - An electronic device dissipating 25 W has a mass...Ch. 4.5 - Prob. 87PCh. 4.5 - 4–88 In a manufacturing facility, 5-cm-diameter...Ch. 4.5 - Prob. 89PCh. 4.5 - Is the metabolizable energy content of a food the...Ch. 4.5 - Is the number of prospective occupants an...Ch. 4.5 - Prob. 92PCh. 4.5 - Prob. 93PCh. 4.5 - Consider two identical 80-kg men who are eating...Ch. 4.5 - A 68-kg woman is planning to bicycle for an hour....Ch. 4.5 - A 90-kg man gives in to temptation and eats an...Ch. 4.5 - A 60-kg man used to have an apple every day after...Ch. 4.5 - Consider a man who has 20 kg of body fat when he...Ch. 4.5 - Consider two identical 50-kg women, Candy and...Ch. 4.5 - Prob. 100PCh. 4.5 - Prob. 101PCh. 4.5 - Prob. 102PCh. 4.5 - Prob. 103PCh. 4.5 - Prob. 104PCh. 4.5 - Prob. 105PCh. 4.5 - Prob. 106PCh. 4.5 - Prob. 107RPCh. 4.5 - Consider a pistoncylinder device that contains 0.5...Ch. 4.5 - Air in the amount of 2 lbm is contained in a...Ch. 4.5 - Air is expanded in a polytropic process with n =...Ch. 4.5 - Nitrogen at 100 kPa and 25C in a rigid vessel is...Ch. 4.5 - Prob. 112RPCh. 4.5 - Prob. 113RPCh. 4.5 - Prob. 114RPCh. 4.5 - 4–115 A mass of 12 kg of saturated...Ch. 4.5 - Prob. 116RPCh. 4.5 - Prob. 117RPCh. 4.5 - Prob. 118RPCh. 4.5 - Prob. 119RPCh. 4.5 - Prob. 120RPCh. 4.5 - Prob. 121RPCh. 4.5 - Prob. 122RPCh. 4.5 - Prob. 123RPCh. 4.5 - Prob. 124RPCh. 4.5 - Prob. 125RPCh. 4.5 - Prob. 126RPCh. 4.5 - Prob. 127RPCh. 4.5 - Prob. 128RPCh. 4.5 - A well-insulated 3-m 4m 6-m room initially at 7C...Ch. 4.5 - Prob. 131RPCh. 4.5 - Prob. 133RPCh. 4.5 - Prob. 134RPCh. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - Prob. 137RPCh. 4.5 - Prob. 138RPCh. 4.5 - A pistoncylinder device initially contains 0.35 kg...Ch. 4.5 - Prob. 140RPCh. 4.5 - 4–141 One kilogram of carbon dioxide is compressed...Ch. 4.5 - Prob. 142RPCh. 4.5 - Prob. 143RPCh. 4.5 - Prob. 144FEPCh. 4.5 - A 3-m3 rigid tank contains nitrogen gas at 500 kPa...Ch. 4.5 - Prob. 146FEPCh. 4.5 - A well-sealed room contains 60 kg of air at 200...Ch. 4.5 - Prob. 148FEPCh. 4.5 - A room contains 75 kg of air at 100 kPa and 15C....Ch. 4.5 - A pistoncylinder device contains 5 kg of air at...Ch. 4.5 - Prob. 151FEPCh. 4.5 - Prob. 152FEPCh. 4.5 - A 2-kW electric resistance heater submerged in 5...Ch. 4.5 - 1.5 kg of liquid water initially at 12C is to be...Ch. 4.5 - An ordinary egg with a mass of 0.1 kg and a...Ch. 4.5 - 4–156 An apple with an average mass of 0.18 kg and...Ch. 4.5 - A 6-pack of canned drinks is to be cooled from 18C...Ch. 4.5 - An ideal gas has a gas constant R = 0.3 kJ/kgK and...Ch. 4.5 - Prob. 159FEPCh. 4.5 - Prob. 161FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A distillation column with a total of 13 actual stages (including a partial condenser) is used to perform a separation which requires 7 ideal stages. Calculate the overall column efficiency, and report your answer in %arrow_forward6. Consider a 10N step input to the mechanical system shown below, take M = 15kg, K = 135N/m, and b = 0.4 Ns/m. (a) Assume zero initial condition, calculate the (i) System pole (ii) System characterization, and (iii) The time domain response (b) Calculate the steady-state value of the system b [ www K 个 х M -F(+)arrow_forward2. Solve the following linear time invariant differential equations using Laplace transforms subject to different initial conditions (a) y-y=t for y(0) = 1 and y(0) = 1 (b) ÿ+4y+ 4y = u(t) for y(0) = 0 and y(0) = 1 (c) y-y-2y=0 for y(0) = 1 and y(0) = 0arrow_forward
- 3. For the mechanical systems shown below, the springs are undeflected when x₁ = x2 = x3 = 0 and the input is given as fa(t). Draw the free-body diagrams and write the modeling equations governing each of the systems. K₁ 000 K₂ 000 M₁ M2 -fa(t) B₂ B₁ (a) fa(t) M2 K₂ 000 B K₁ x1 000 M₁ (b)arrow_forwardThis question i m uploading second time . before you provide me incorrect answer. read the question carefully and solve accordily.arrow_forward1. Create a table comparing five different analogous variables for translational, rotational, electrical and fluid systems. Include the standard symbols for each variable in their respective systems.arrow_forward
- 2) Suppose that two unequal masses m₁ and m₂ are moving with initial velocities v₁ and v₂, respectively. The masses hit each other and have a coefficient of restitution e. After the impact, mass 1 and 2 head to their respective gaps at angles a and ẞ, respectively. Derive expressions for each of the angles in terms of the initial velocities and the coefficient of restitution. m1 m2 8 m1 m2 βarrow_forward4. Find the equivalent spring constant and equivalent viscous-friction coefficient for the systems shown below. @ B₁ B₂ H B3 (b)arrow_forward5. The cart shown below is inclined 30 degrees with respect to the horizontal. At t=0s, the cart is released from rest (i.e. with no initial velocity). If the air resistance is proportional to the velocity squared. Analytically determine the initial acceleration and final or steady-state velocity of the cart. Take M= 900 kg and b 44.145 Ns²/m². Mg -bx 2 отarrow_forward
- 9₁ A Insulated boundary Insulated boundary dx Let's begin with the strong form for a steady-state one-dimensional heat conduction problem, without convection. d dT + Q = dx dx According to Fourier's law of heat conduction, the heat flux q(x), is dT q(x)=-k dx. x Q is the internal heat source, which heat is generated per unit time per unit volume. q(x) and q(x + dx) are the heat flux conducted into the control volume at x and x + dx, respectively. k is thermal conductivity along the x direction, A is the cross-section area perpendicular to heat flux q(x). T is the temperature, and is the temperature gradient. dT dx 1. Derive the weak form using w(x) as the weight function. 2. Consider the following scenario: a 1D block is 3 m long (L = 3 m), with constant cross-section area A = 1 m². The left free surface of the block (x = 0) is maintained at a constant temperature of 200 °C, and the right surface (x = L = 3m) is insulated. Recall that Neumann boundary conditions are naturally satisfied…arrow_forward1 - Clearly identify the system and its mass and energy exchanges between each system and its surroundings by drawing a box to represent the system boundary, and showing the exchanges by input and output arrows. You may want to search and check the systems on the Internet in case you are not familiar with their operations. A pot with boiling water on a gas stove A domestic electric water heater A motor cycle driven on the roadfrom thermodynamics You just need to draw and put arrows on the first part a b and carrow_forward7. A distributed load w(x) = 4x1/3 acts on the beam AB shown in Figure 7, where x is measured in meters and w is in kN/m. The length of the beam is L = 4 m. Find the moment of the resultant force about the point B. w(x) per unit length L Figure 7 Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license