
Consider two chemical changes: one occurring at a tetrahedral
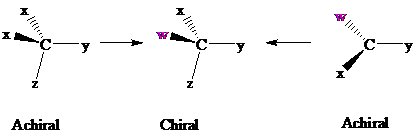
Both transformations convert C in each achiral reactant to a chirality center in the product. The two achiral reactants are classified as prochiral. C is a prochiralitycenter in
In achiral molecules with tetrahedral prochiralitycenters, substitution of one of the two x groups by w gives the enantiomer of the product that results from substitution of the other. The two x groups occupy mirror-image sites and are enantiotopic.
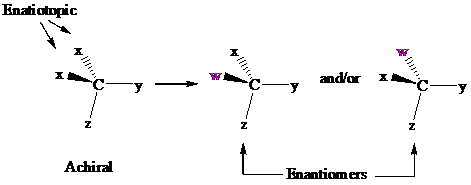
Enantiotopic groups are designated as pro-R or pro-S by a modification of Cahn–Ingold– Prelog notation. One is assigned a higher priority than the other without disturbing the priorities of the remaining groups, and the R,S configuration of the resulting chirality center is determined in the usual way. If it is R, the group assigned the higher rank is pro-R. If S, this group is pro-S. Ethanol and citric acid illustrate the application of this notation to two prochiral molecules.
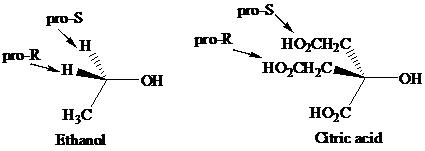
Citric acid played a major role in the development of the concept of prochirality. Its two
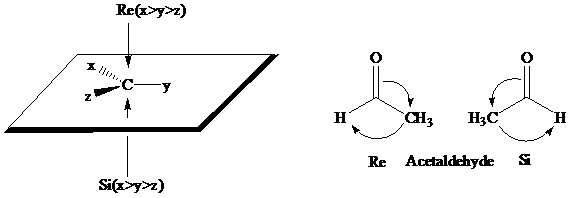
The stereochemical aspects of many enzyme-catalyzed reactions have been determined. Then enzyme alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde by removing the pro-R hydrogen (abbreviated as HR). When the same enzyme catalyzes the reduction of acetaldehyde to ethanol, hydrogen is transferred to the Re face.
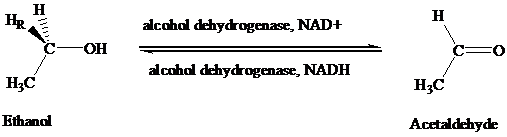
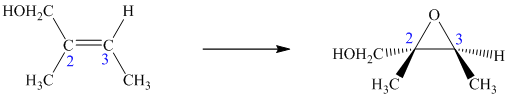
A method for the stereoselective synthesis of chiral
Re Re
Re Si
Si Si
Si Re
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- utron eutro cle TH tro (Na (b) Atoms are said to be electrically neutral. Explain. (c) Distinguish between the following: (i) Atomic number and mass number. (ii) Mass number and relative atomic mass. 2. An isotope Q, has 18 neutrons a mass number of 34. (a) (i) Draw the atomic structure of Q. (ii) Write its electron arrangement (b) To which period and group does Q belong? Explain your answer. (c) How does Q form its ion? Explain. 3. (a) Determine the relative atomic mass of the following elements = compositions occur in the proportions given. (i) Neon 20 21 22. Ne (90.92%), 10Ne (0.26%), and 10Ne (8.82%) (ii) Argon 36 38 40 18 Ar (0.34%), 18 Ar (0.06%) and 18 Ar (99.6%)arrow_forwardIn the normal hydrogen electrode, the balance potential difference in the interface is this, the maximum potential is 5 mV. Explain briefly.arrow_forwardThe electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The overvoltage n will be 0.005 - (-0.118) = 0.123 V. Is it correct?arrow_forward
- In the electrode Pt, H2(1 atm) | H+(a=1), if the electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The current voltage will be 0.005 - (-0.118) = 0.123 V ¿Correcto?arrow_forwardIn the electrode Pt, H2(1 atm) | H+(a=1) at 298K is 0.79 mA cm-2. If the balance potential of the electrode is -0.118 V and the potential difference of the interface is +5 mV. Determine its potential.arrow_forwardIn one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.arrow_forward
- In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forwardIf the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forward
- Topic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forward

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


