
Consider two chemical changes: one occurring at a tetrahedral
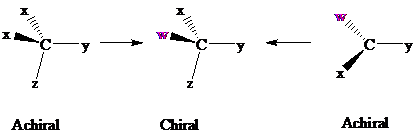
Both transformations convert C in each achiral reactant to a chirality center in the product. The two achiral reactants are classified as prochiral. C is a prochiralitycenter in
In achiral molecules with tetrahedral prochiralitycenters, substitution of one of the two x groups by w gives the enantiomer of the product that results from substitution of the other. The two x groups occupy mirror-image sites and are enantiotopic.
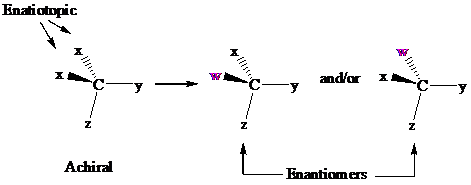
Enantiotopic groups are designated as pro-R or pro-S by a modification of Cahn–Ingold– Prelog notation. One is assigned a higher priority than the other without disturbing the priorities of the remaining groups, and the R,S configuration of the resulting chirality center is determined in the usual way. If it is R, the group assigned the higher rank is pro-R. If S, this group is pro-S. Ethanol and citric acid illustrate the application of this notation to two prochiral molecules.
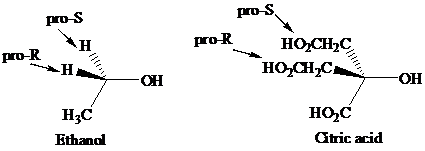
Citric acid played a major role in the development of the concept of prochirality. Its two
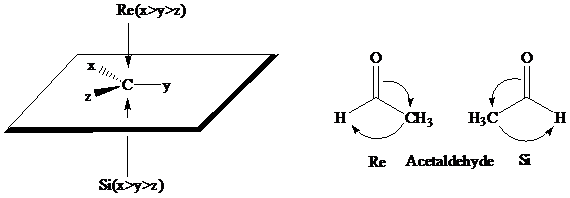
The stereochemical aspects of many enzyme-catalyzed reactions have been determined. Then enzyme alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde by removing the pro-R hydrogen (abbreviated as HR). When the same enzyme catalyzes the reduction of acetaldehyde to ethanol, hydrogen is transferred to the Re face.
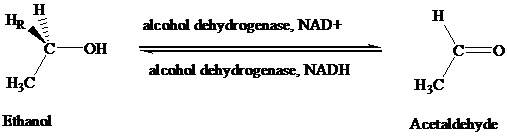
When the achiral dione shown (below left) was incubated in water with baker’s yeast, reduction of one of the
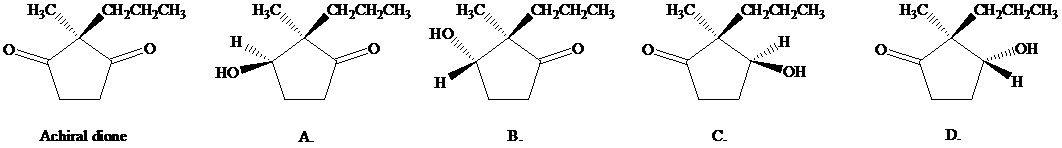
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- 7. Which of the following drawings represents the enantiomer of compound X? CH, H,C-C-CH, ОН CH, CH, OH OH OH CH, H,C-C-CH, H,C-C-CH, H,C HO, ÓH OH A. В. С. 8. Which of the compounds below is (are) chiral? Br А. А В. В С. С Br Br Br D. D E. A and B F. B and D Br Br Br А. В. D.arrow_forward1. Ketones react with sodium acetylide (the sodium salt of acetylene, Na“:C=CH) to give alcohols. For example: OH 2 H;0 a. Is the product chiral? b. Draw both major and minor reaction products, assuming that the reaction takes place preferentially from the Re face of the carbonyl group. Is the product mixture optically active? Explain.arrow_forward1) For each pair of structures, determine if they are identical (I), resonance forms (RF), structural isomers (S), conformers (C), enantiomers (E), diastereomers (D), or None (N). b) F H OCH 3 H3CO H F Om Ω d) f) CI CI LL www LL CH3 H CI CH H H H H3C CI CH3arrow_forward
- Explain why they would have different chemical properties.arrow_forwardCH3 CH3 CH3 CH3 CH3 H Br H Br H Br- H H. H. H 'H. H3C CH3 ČH3 Br 2-bromobutane a b The wedge-dash representation of 2-bromobutane is shown. What is the orientation of the chiral center(s)? Write the orientation as in the following examples, (2S) or (2R,3S), not 2s or 2R,3S. Be sure to include the parentheses and the number, and comma as necessary. Which of the Newman structures is equivalent to the wedge-dash structure? Note that the equivalent Newman structure will not necessarily have the same conformation as the wedge-dash structure.arrow_forwardWhich of the following molecules is/ are chiral? بر H3C H3C H₂C CI CH₂OH H CH3 Main Content CF3 CH3 Br millBrarrow_forward
- What are the configurations of the chiral carbon atoms in this compound? Carbon atom number 1 is the carbon containing the aldehyde. H H HO- CHO OH -OH -H CH₂OH (2R 3R,4S) O (25,35,45) Ⓒ (25,35,4R) O (2R,35,45) O (2S,3R.4R) O (2R 3R,4R) Ⓒ(2S,3R.4S) O (2R,3S,4R)arrow_forwardQ2 Give the stereochemical relationships between each pair of structures. Examples are same compound, structural isomers, enantiomers, diastereomers fa CH. OH HO- OR HO CH, CH. CH,arrow_forward5aarrow_forward
- Please draw all four bonds at chiral centers. i Draw curved arrows to show the reaction between the two compounds. DC 12D \ Z OO +1: H C N O S F P Cl Br Iarrow_forwardWhich of these molecules have chiral center?arrow_forwardNemotin is a compound that was first isolated from the fungi Poria tenuis and Poria corticola in the 1940s and was shown to possess potent antibacterial activity. (Org. Biomol. Chem. 2010, 8, 811-821). However, its structure was not verified until it was made in the laboratory much more recently. :ö H H-C-C-H H C=c=C H Nemotin Determine the hybridization state of each carbon atom in nemotin. ミ-0三O-O三arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


