
Concept explainers
Give the IUPAC name for each compound.
a.
b.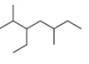
c.
d.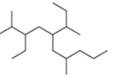
(a)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.8P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is
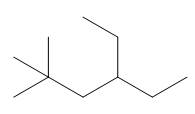
Figure 1
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
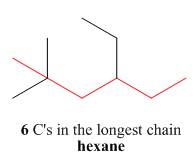
Figure 2
The second step is numbering of chain.
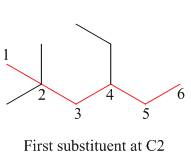
Figure 3
The third step is naming and numbering of substituents.
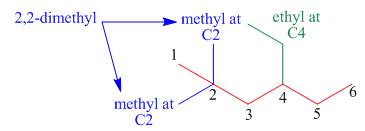
Figure 4
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
(b)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.8P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is,
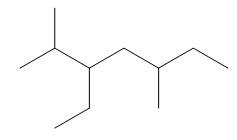
Figure 5
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
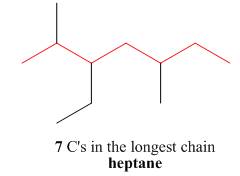
Figure 6
The second step is numbering of chain.
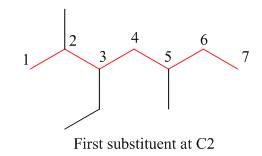
Figure 7
The third step is naming and numbering of substituents.
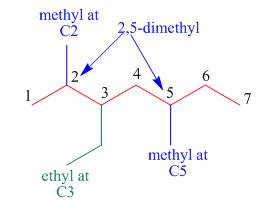
Figure 8
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
(c)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.8P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is
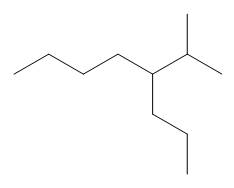
Figure 9
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
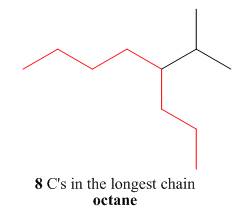
Figure 10
The second step is numbering of chain.
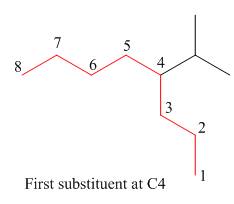
Figure 11
The third step is naming and numbering of substituents.
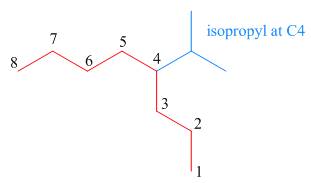
Figure 12
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
(d)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.8P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is,
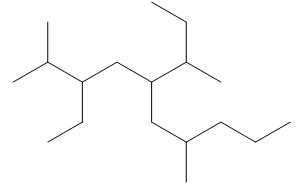
Figure 13
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
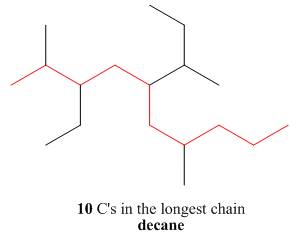
Figure 14
The second step is numbering of chain.
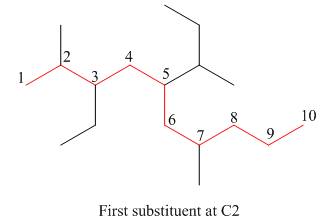
Figure 15
The third step is naming and numbering of substituents.
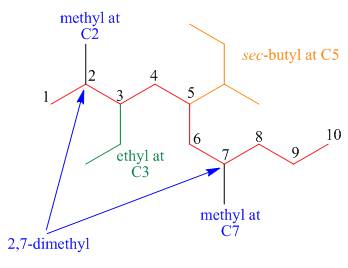
Figure 16
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 4 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry - 4th edition
Organic Chemistry (8th Edition)
Microbiology with Diseases by Body System (5th Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology Fundamentals: A Clinical Approach
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

