
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.48P
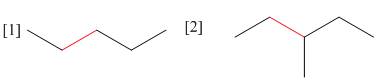
(a) Using Newman projections, draw all staggered and eclipsed conformations that
result from rotation around the bond highlighted in red in each molecule; (b) draw a graph of energy versus dihedral angle for rotation around this bond.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 4 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 4 - Prob. 4.1PCh. 4 - Problem 4.2 Which of the following is not another...Ch. 4 - Problem 4.3 Draw the five constitutional isomers...Ch. 4 - Prob. 4.4PCh. 4 - Prob. 4.5PCh. 4 - Draw the five constitutional isomers that have...Ch. 4 - Problem 4.7 Give the IUPAC name for each...Ch. 4 - Give the IUPAC name for each compound. a....Ch. 4 - Problem 4.9 Give the structure corresponding to...Ch. 4 - Prob. 4.10P
Ch. 4 - Give the IUPAC name for each compound.Ch. 4 - Give the structure corresponding to each IUPAC...Ch. 4 - Arrange the following compounds in order of...Ch. 4 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4 - Prob. 4.15PCh. 4 - Prob. 4.16PCh. 4 - Problem 4.17 a. Draw the three staggered and...Ch. 4 - Problem 4.18 Rank the following conformations in...Ch. 4 - Problem 4.19 Consider rotation around the...Ch. 4 - Calculate the destabilization present in each...Ch. 4 - Problem 4.21 Classify the ring carbons as up or...Ch. 4 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4 - Draw a second chair conformation for each...Ch. 4 - Problem 4.24 Draw both conformations for and...Ch. 4 - Problem 4.25 Draw the structure for each compound...Ch. 4 - For cis-1, 3-diethylcyclobutane, draw a a...Ch. 4 - Prob. 4.27PCh. 4 - Problem 4.28 Consider .
Draw structures f or the...Ch. 4 - Problem 4.29 Draw a chair conformation of...Ch. 4 - Prob. 4.30PCh. 4 - Draw the products of each combustion reaction.Ch. 4 - Explain why beeswax is insoluble in H2O, slightly...Ch. 4 - Prob. 4.33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 - Consider the substituted cyclohexane shown in the...Ch. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - 4.38 Give the IUPAC name for each compound.
a. c....Ch. 4 - 4.39 Give the structure and IUPAC name for each of...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - Prob. 4.41PCh. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 4.43PCh. 4 - Prob. 4.44PCh. 4 - 4.45 Which conformation in each pair is higher in...Ch. 4 - 4.46 Considering rotation around the bond...Ch. 4 - Prob. 4.47PCh. 4 - 4.48 (a) Using Newman projections, draw all...Ch. 4 - 4.49 Label the sites of torsional and steric...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - 4.56 Convert each of the following structures into...Ch. 4 - Prob. 4.57PCh. 4 - Prob. 4.58PCh. 4 - 4.59 Classify each pair of compounds as...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 4.61PCh. 4 - 4.62 Draw the three constitutional isomers having...Ch. 4 - Prob. 4.63PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 4.66PCh. 4 - Prob. 4.67PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 4.69PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 4.71PCh. 4 - Prob. 4.72PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License