
Concept explainers
Interpretation: The weakest acid and weakest base in below table should be determined.
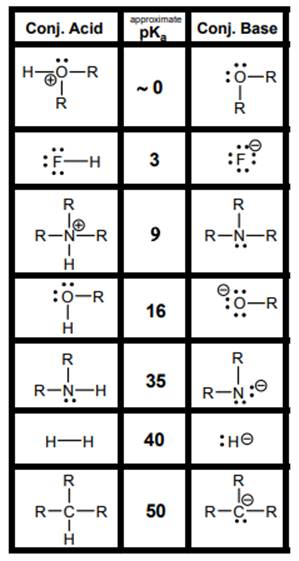
Concept introduction: According to Bronsted-Lowry concept, substance that donates proton is termed as acid while that accepts or gains protons is called base. Species formed after loss of protons from acids are known as their respective conjugate bases whereas conjugate acid is produced by addition of protons to base. Strength of conjugate acids and conjugate bases are inversely related to strengths of their respective bases and acids.
According to Lewis concept, substance that donates electron pair is termed as base while that accepts or gains electron pair is called acid. For example,
According to Arrhenius concept, substances that donate hydrogen ions in solutions are known as acids while bases are substances that release hydroxide ions in solutions.
Trending nowThis is a popular solution!

Chapter 4 Solutions
Custom eBook for Organic Chemistry
- Feedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when acetophenone reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of N-(4-methylphenyl)acetamide with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained from the reaction of 4-(trifluoromethyl)benzonitrile with a sulfonitric mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained in the reaction of p-Toluidine with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 4-methylbenzonitrile with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

