
Draw the structure corresponding to each IUPAC name.
a.
b.
c.
d. cyclobutylcycloheptane
e.
f.
g
h.
i.
j.
(a)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.41P
The structure corresponding to
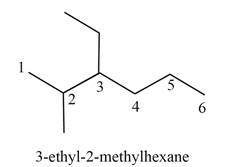
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
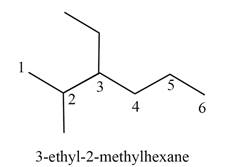
Figure 1
The structure corresponding to the given name is shown in Figure 1.
(b)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
4. First identify the word root for the given compound.
5. The suffix used in the compound like –ene.
6. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.41P
The structure corresponding to
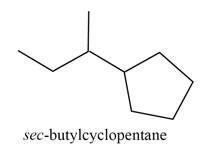
Explanation of Solution
The given IUPAC name is
The given name is

Figure 2
The structure corresponding to the given name is shown in Figure 2.
(c)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.41P
The structure corresponding to
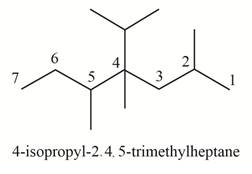
Explanation of Solution
The given IUPAC name is
The given name is
Thus, the correct structure of

Figure 3
The structure corresponding to the given name is shown in Figure 3.
(d)
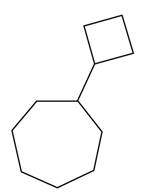
Interpretation: The structure corresponding to cyclobutylcycloheptane is to be drawn.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.41P
The structure corresponding to cyclobutylcycloheptane is shown as,
Explanation of Solution
The given IUPAC name is cyclobutylcycloheptane. The given name is cyclobutylcycloheptane. The word root used in this is hept. It means structure contains seven carbon atoms. The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order. Therefore, the name suggests that one cyclobutyl substituents is attached to the carbon atom of cycloheptane.
Thus, the correct structure of cyclobutylcycloheptane is shown below.
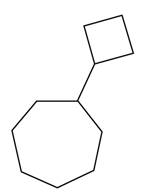
Figure 4
The structure corresponding to the given name is shown in Figure 4.
(e)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.41P
The structure corresponding to
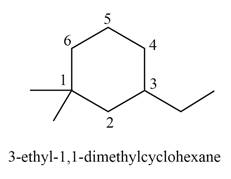
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 5
The structure corresponding to the given name is shown in Figure 5.
(f)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.41P
The structure corresponding to
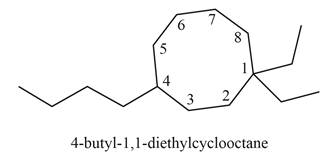
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 6
The structure corresponding to the given name is shown in Figure 6.
(g)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.41P
The structure corresponding to
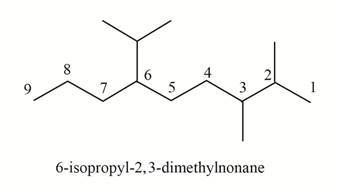
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 7
The structure corresponding to the given name is shown in Figure 7.
(h)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.41P
The structure corresponding to
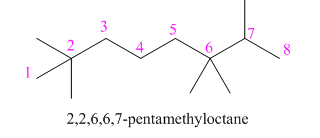
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 8
The structure corresponding to the given name is shown in Figure 8.
(i)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
4. First identify the word root for the given compound.
5. The suffix used in the compound like –ene.
6. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.41P
The structure corresponding to
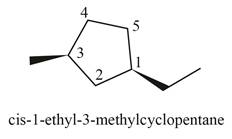
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
7. First identify the word root for the given compound.
8. The suffix used in the compound like –ene.
9. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
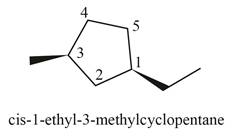
Figure 9
The structure corresponding to the given name is shown in Figure 9.
(j)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
10. First identify the word root for the given compound.
11. The suffix used in the compound like –ene.
12. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.41P
The structure corresponding to
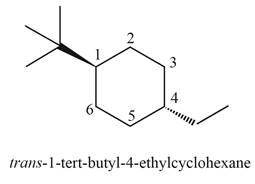
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
13. First identify the word root for the given compound.
14. The suffix used in the compound like –ene.
15. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of

Figure 10
The structure corresponding to the given name is shown in Figure 10.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry-Package(Custom)
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Applications and Investigations in Earth Science (9th Edition)
Campbell Essential Biology (7th Edition)
HUMAN ANATOMY
Concepts of Genetics (12th Edition)
Fundamentals Of Thermodynamics
- 75.0 grams of an unknown metal was heated to 95.0°C, it was then placed into 150.0 grams of water at23.1°C, when the metal and water reached thermal equilibrium, the temperature was 27.8°C. Calculatethe specific heat of the metal. (Assume that the specific heat of water is 4.18 J/g °C)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardA 25.0 g sample of water was cooled from 23.9°C to 12.7°C, how much heat was released? (Assume thatthe specific heat of water is 4.18 J/g °C)arrow_forward
- Zeolites: environmental applications.arrow_forward" is The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3 best described as a hybrid of several contributing resonance forms, two of which are shown here. HO :0: :Ö: HO + Bicarbonate is crucial for the control of body pH (for example, blood pH: 7.4). A more self-indulgent use is in baking soda, where it serves as a source of CO2 CO₂ 2 gas, which gives bread and pastry their fluffy constituency. (i) Draw at least one additional resonance form. = (ii) Using curved "electron-pushing" arrows, show how these Lewis structures may be interconverted by movement of electron pairs. (iii) Determine which form or forms will be the major contributor(s) to the real structure of bicarbonate, explaining your answer on the basis of the criteria in Section 1-5.arrow_forwardWhich of these is the best use of a volumetric flask? measuring how much liquid it contains delivering a precise amount of liquid to another container holding solutions making solutions of precise concentrationarrow_forward
- You're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





