
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.21P
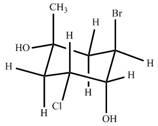
Classify the ring carbons as up
. 
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How to draw this claisen condensation reaction mechanisms/
Write all of Me Possible Products For each
Of the Following reactions. In each case identity
all pains of enantiomers, all digsterzoners and
all Meso compounds
9.
11-60
11-0-11
V-G
Η
Η
H
~ C-11 +HB+ -
1
H
b.
पन्ना
171-0-11
H-C-H
Н
C-C=c-call +HBr Perendez
==
How can i draw the mechanisms for this molecule?
Chapter 4 Solutions
Organic Chemistry-Package(Custom)
Ch. 4 - Prob. 4.1PCh. 4 - Prob. 4.2PCh. 4 - (a) Classify the carbon atoms in each compound as...Ch. 4 - Problem 3.3 Classify a carbon atom by the number...Ch. 4 - Problem 4.3 Draw the five constitutional isomers...Ch. 4 - Prob. 4.6PCh. 4 - Prob. 4.7PCh. 4 - Draw the five constitutional isomers that have...Ch. 4 - Give the IUPAC name for each compound. a. c. b. ...Ch. 4 - Give the IUPAC name for each compound. a....
Ch. 4 - Problem 4.9 Give the structure corresponding to...Ch. 4 - Prob. 4.12PCh. 4 - Give the IUPAC name for each compound.Ch. 4 - Give the structure corresponding to each IUPAC...Ch. 4 - Arrange the following compounds in order of...Ch. 4 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4 - Prob. 4.17PCh. 4 - a.Draw the three staggered and three eclipsed...Ch. 4 - Problem 4.19 Consider rotation around the...Ch. 4 - Calculate the destabilization present in each...Ch. 4 - Problem 4.21 Classify the ring carbons as up or...Ch. 4 - Draw a second chair conformation for each...Ch. 4 - Prob. 4.23PCh. 4 - Problem 4.25 Draw the structure for each compound...Ch. 4 - For cis-1, 3-diethylcyclobutane, draw a a...Ch. 4 - Prob. 4.26PCh. 4 - Problem 4.28 Consider .
Draw structures f or the...Ch. 4 - Problem 4.29 Draw a chair conformation of...Ch. 4 - Prob. 4.29PCh. 4 - Draw the products of each combustion reaction.Ch. 4 - Explain why beeswax is insoluble in H2O, slightly...Ch. 4 - Prob. 4.32PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 - Consider the substituted cyclohexane shown in the...Ch. 4 - Prob. 4.35PCh. 4 - 3.31 For each alkane: (a) classify each carbon...Ch. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Give the IUPAC name for each compound. a. h.k....Ch. 4 - 4.39 Give the structure and IUPAC name for each of...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - Prob. 4.42PCh. 4 - Prob. 4.43PCh. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 4.45PCh. 4 - Prob. 4.46PCh. 4 - 4.45 Which conformation in each pair is higher in...Ch. 4 - Considering rotation around the bond highlighted...Ch. 4 - Prob. 4.49PCh. 4 - Prob. 4.50PCh. 4 - 4.49 Label the sites of torsional and steric...Ch. 4 - Prob. 4.52PCh. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - 4.56 Convert each of the following structures into...Ch. 4 - Prob. 4.59PCh. 4 - Prob. 4.60PCh. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 4.62PCh. 4 - Prob. 4.63PCh. 4 - 4.62 Draw the three constitutional isomers having...Ch. 4 - Prob. 4.65PCh. 4 - Prob. 4.66PCh. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Which of the following compounds are lipids?Ch. 4 - Prob. 4.69PCh. 4 - Prob. 4.70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 4.72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 4.74PCh. 4 - Prob. 4.75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forwardhelp draw any straightchain moleculearrow_forward
- How to do the mechanism drawn for the reactionarrow_forwardPlease provide the mechanism for this reacitonarrow_forwardQuestion 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forward
- Question 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forwardA. Add Water to below compound which 2-methyl 2-butene (addition Reaction) H₂C CH₂ CH, + H₂O-> ? Major product? Minor product? B. Add Bromine to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + Br₂→ ? Major product and Minor product both are same in this? C. Add Hydrogen Bromide to the compound which 2-methyl 2-butene (addition Reaction) H,C CH₂ CH₂ + HBr Major product? Minor product? D. Add Hydrogen to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + H₂ Major product and Minor product both are same in this?arrow_forward36) Complete the following multi-step reactions showing applications of enolate ions arising from ketones, esters, malonic ester, and keto ester, etc. (30 pts) (1) A NaOH, H₂O+ heat A NaOEt EtO OEt (11) EOH, H+ H. B LDA, H₂O+ -78°C B (i) NaOMe, Et-Br (ii) H₂O+, heat EtOOC (III) COOEt B A (i) NaOEt LiAlH 4-bromo-2-butene H₂O+ (ii) H3O+, heat Write the mechanism for Aldol Condensation (I A or B), and Claisen Condensation (II A).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY