
Concept explainers
(a)
The plot of the logarithm of the rate of recrystallization versus the inverse of the absolute temperature and to check whether it is linear or not.
Answer to Problem 4.19P
The graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature is shown in figure (1) and it is linear.
Explanation of Solution
Given:
The given table is,
| S. No. | Temperature |
Time (Seconds) |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
392 | |
| |
|
|
Calculation:
Draw the table to plot the graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature.
| S. No. | Temperature |
|
Time
|
Rate
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
392 | |
|
|
|
| |
|
|
|
|
|
The graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature is shown below.
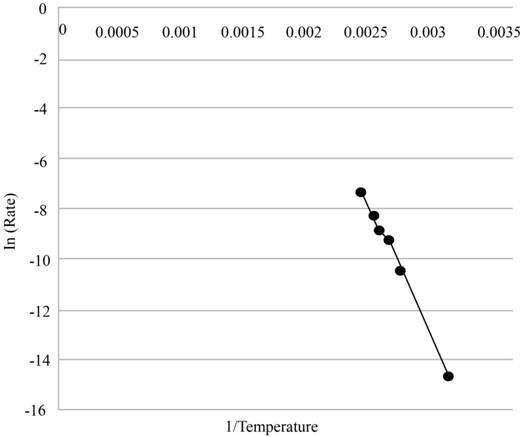
Figure (1)
Conclusion:
Therefore, the graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature is shown in figure (1) and it is linear.
(b)
The activation enthalpy for the recrystallization of copper.
Answer to Problem 4.19P
The activation enthalpy for the recrystallization of copper is
Explanation of Solution
Formula Used,
The activation enthalpy for the recrystallization of copper is given by,
Here,
Calculation:
The activation enthalpy for the recrystallization of copper is calculated as,
Substitute
Conclusion:
Therefore, the activation enthalpy for the recrystallization of copper is
(c)
The reason for activation enthalpy of recrystallization of copper being less than the activation enthalpy for vacancy diffusion.
Answer to Problem 4.19P
The activation enthalpy for the recrystallization is less than the activation enthalpy for vacancy diffusion because during the process of recrystallization, the size of grains increases and the vacancy site decreases.
Explanation of Solution
Introduction:
The Gibbs free energy is the energy that is available or free, to do work under conditions of constant pressure and temperature.
The expression for the Gibb’s free energy is given by,
Here,
The term
Here,
During the process of recrystallization, the size of the grain changes to form a new crystal structure. By this process the grain size changes its size by diffusing into other grains, thereby increasing the size of the grains. When the size of the grain increases, the possibility for the vacancy site decreases; that’s why the activation enthalpy of recrystallization of copper is less than the activation enthalpy for vacancy diffusion.
Conclusion:
Therefore, the activation enthalpy for the recrystallization is less than the activation enthalpy for vacancy diffusion because during the process of recrystallization, the size of grains increases and the vacancy site decreases.
(d)
The temperature at which the copper completely crystallizes in
Answer to Problem 4.19P
The temperature at which the copper completely crystallizes in
Explanation of Solution
Formula Used,
The logarithmic rate of crystallization is given by,
Here,
Calculation:
The logarithmic rate of crystallization is calculated as,
Substitute
Drop a perpendicular from
Conclusion:
Therefore, the temperature at which the copper completely crystallizes in
Want to see more full solutions like this?
Chapter 4 Solutions
Materials Science And Engineering Properties
- Why is it important for construction project managers to be flexible when dealing with the many variable factors that pop up in a project?arrow_forwardWhat are some reasons for why a company would accelerate a construction project?arrow_forwardFor the design of a shallow foundation, given the following: Soil: ' = 20° c' = 52 kN/m² Unit weight, y = 15 kN/m³ Modulus of elasticity, E, = 1400 kN/m² Poisson's ratio, μs = 0.35 Foundation: L=2m B=1m Df = 1 m Calculate the ultimate bearing capacity. Use the equation: 1 - qu = c' NcFcs Fcd Fcc +qNqFqsFqdFqc + ½√BN√Fãs F√dƑxc 2 For '=20°, Nc = 14.83, N₁ = 6.4, and N₁ = 5.39. (Enter your answer to three significant figures.) qu = kN/m²arrow_forward
- A 2.0 m wide strip foundation carries a wall load of 350 kN/m in a clayey soil where y = 15 kN/m³, c' = 5.0 kN/m² and ' = 23°. The foundation depth is 1.5 m. For ' = 23°: Nc = 18.05; N₁ = 8.66; Ny = = = 8.20. Determine the factor of safety using the equation below. qu= c' NcFcs FcdFci+qNqFqsFq 1 F + gd. 'qi 2 ·BN√· FF γί Ysyd F (Enter your answer to three significant figures.) FS =arrow_forward2P -1.8 m- -1.8 m- -B Wo P -1.8 m- Carrow_forwardPart F: Progressive activity week 7 Q.F1 Pick the rural location of a project site in Victoria, and its catchment area-not bigger than 25 sqkm, and given the below information, determine the rainfall intensity for ARI 5, 50, 100 year storm event. Show all the details of the procedure. Each student must propose different length of streams and elevations. Use fig below as a sample only. Pt. E-nt 950 200 P: D-40, PC-92.0 300m 300m 000m PL.-02.0 500m HI-MAGO PLA-M 91.00 To be deemed satisfactory the solution must include: Q.F1.1.Choice of catchment location Q.F1.2. A sketch displaying length of stream and elevation Q.F1.3. Catchment's IFD obtained from the Buro of Metheorology for specified ARI Q.F1.4.Calculation of the time of concentration-this must include a detailed determination of the equivalent slope. Q.F1.5.Use must be made of the Bransby-Williams method for the determination of the equivalent slope. Q.F1.6.The graphical display of the estimation of intensities for ARI 5,50, 100…arrow_forward
- I need help finding: -The axial deflection pipe in inches. -The lateral deflection of the beam in inches -The total deflection of the beam like structure in inches ?arrow_forwardA 2.0 m wide strip foundation carries a wall load of 350 kN/m in a clayey soil where y = 17 kN/m³, c' = 5.0 kN/m² and 23°. The foundation depth is 1.5 m. For o' = 23°: Nc = 18.05; N = 8.66; N = 8.20. Determine the factor of safety using the equation below. 1 qu = c' NcFcs Fed Fci +qNqFqs FqdFqi + ½ BN F√s 1 2 (Enter your answer to three significant figures.) s Fyd Fi FS =arrow_forward1.2 m BX B 70 kN.m y = 16 kN/m³ c' = 0 6'-30° Water table Ysat 19 kN/m³ c' 0 &' = 30° A square foundation is shown in the figure above. Use FS = 6, and determine the size of the foundation. Use the Prakash and Saran theory (see equation and figures below). Suppose that F = 450 kN. Qu = BL BL[c′Nc(e)Fcs(e) + qNg(e)Fcs(e) + · 1 YBN(e) F 2 7(e) Fra(e)] (Enter your answer to two significant figures.) B: m Na(e) 60 40- 20- e/B=0 0.1 0.2 0.3 .0.4 0 0 10 20 30 40 Friction angle, ' (deg) Figure 1 Variation of Na(e) with o' Ny(e) 60 40 20 e/B=0 0.3 0.1 0.2 0.4 0 0 10 20 30 40 Friction angle, ' (deg) Figure 2 Variation of Nye) with o'arrow_forward
- K/S 46. (O المهمات الجديدة 0 المنتهية 12 المغـ ۱۱:۰۹ search ليس لديك اي مهمات ☐ ○ ☑arrow_forwardI need help setti if this problem up and solving. I keep doing something wrong.arrow_forward1.0 m (Eccentricity in one direction only)=0.15 m Call 1.5 m x 1.5m Centerline An eccentrically loaded foundation is shown in the figure above. Use FS of 4 and determine the maximum allowable load that the foundation can carry if y = 18 kN/m³ and ' = 35°. Use Meyerhof's effective area method. For '=35°, N = 33.30 and Ny = 48.03. (Enter your answer to three significant figures.) Qall = kNarrow_forward
 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
