Concept explainers
Interpretation:Difference between Thomson’s and Dalton’s atomic theory needs to be explained.
Concept introduction: Theory is a group of connected ideas or concepts intended to describe something. It involves question- “why” or “how anything/ somethings happens. It is based on inference.
Answer to Problem 1RQ
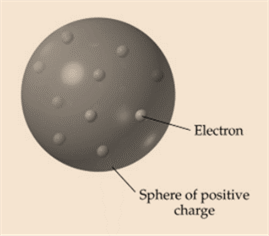
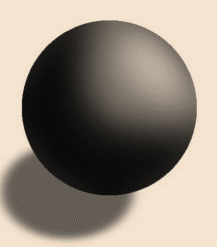
Dalton’s atomic theory was proposed by John Dalton. According to the Dalton’s atomic theory, matter is composed of tiny particles, all atoms of certain element are identical, compound contains more than one element. In J.J. Thomson’s atomic theory, electrons are distributed inside a positive mass like raising in a plum pudding.
Explanation of Solution
Scientist John Dalton and Scientist JJ Thompson proposed different models of an atom.Difference between Thomson’s atomic theoryand Dalton’s atomic theory is mentioned below:
| Thomson’s atomic theory | Dalton’s atomic theory |
| Thomson’s model of the atom includes electrons in a plum pudding model. | Dalton’s model does not and is more of a solid sphere. |
| Tiny negative electrons were distributed in a positive (+ve) mass inside the atom. The electrons were like negative (-ve) raising in a positive(+ve) plum pudding dough. | Atoms were tiny, hard spheres that vary in size as well as mass, but can’t be split into smaller pieces. |
| Picture of plum pudding model proposed by JJ Thompson:
| Picture of solid sphere model proposed by the scientist John Dalton:
|
Thomson’s atomic theoryand Dalton’s atomic theory are not the same.
Chapter 3 Solutions
World of Chemistry, 3rd edition
- x 人 3. Fill in the missing information HCN, NaCN A. 3 D. E. H. P H₂O' B. W F. Chaug Br H. NOCHON H20 H* 左 NC. OH (Ph)3P Nech MeOH,HCI OH G. Ph DELL DOWarrow_forwardBe a. Draw an arrow pushing mechanism for the following. OH O HO. W X J DELL OH H₂O HO 8. Walmart wil FS F6 F7 B F8 prt sc home F9 F10 F11 end F12 inse A & * +arrow_forwardPredict the product of this organic reaction: H+ + HO OH A P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. X 5arrow_forward
- Which synthesis of a Grignard reagent would fail to occur as written? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardDraw the structure of the major organic product(s) of the reaction. H3C 1. DIBAH, toluene -CH3 + 2. H3O DIBAH = diisobutylaluminum hydride, [(CH3)2CHCH2]2AIHarrow_forwardWhich of the following is not an intermediate of the reaction below? Why is the correct answer C? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forward
- Which of the following is the product of the reaction between acetone, CH3COCH3 and methyl amine, CH3NH2? Why is the correct answer A? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the reaction shown below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWrite the systematic name of each organic molecule: structure name П O ☐ O ☐ Oarrow_forward
- The 13C NMR signal for which of the indicated carbons will occur at the frequency (most deshielded)? Why is the correct answer E? Please explain what is happening. Please include a detailed explanation needed to understand the or question.arrow_forwardWhich of the following reagents best achieves the reaction shown below? Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the following reaction sequence? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY