
Concept explainers
(a)
Interpretation:
Using the
Concept Introduction:
Every matter is made of chemicals. Chemistry is very important for the study of everything. Chemistry is all about how something interacts to affect the structure, composition, as well as properties of substances.
(a)
Answer to Problem 59A
The element with the given chemical symbol,
Explanation of Solution
Protons are defined as positively charged
Electrons are negatively charged subatomic particles found orbiting the nucleus of an atom.
For neutral atom, the number of Electron = number of protons.
Neutrons are subatomic particles found in the nucleus of an atom which is neutral.
An atom has a nucleus with a positive charge due to its protons and has electrons in the space surrounding the nucleus at a relatively large distance from it.
For
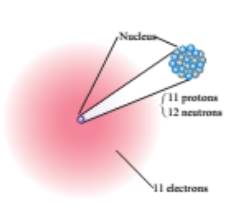
From the given figure, the first element contains
The atomic number of the element is
Hence, the first element is
(b)
Interpretation:
Using the
Concept Introduction:
Every matter is made of chemicals. Chemistry is very important for the study of everything. Chemistry is all about how something interacts to affect the structure, composition, as well as properties of substances.
(b)
Answer to Problem 59A
The elements with the chemical symbol and atomic number and mass number -
Explanation of Solution
Protons are defined as positively charged
Electrons are negatively charged subatomic particles found orbiting the nucleus of an atom.
For neutral atom, the number of Electron = number of protons.
Neutrons are subatomic particles found in the nucleus of an atom which is neutral.
An atom has a nucleus with a positive charge due to its protons and has electrons in the space surrounding the nucleus at a relatively large distance from it.
For
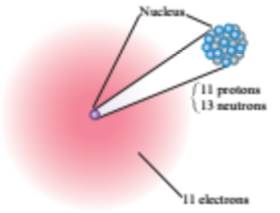
From the given figures
The second element contains
The atomic number of the element is
Hence, the second element is
(c)
Interpretation:
Using the
Concept Introduction:
Every matter is made of chemicals. Chemistry is very important for the study of everything. Chemistry is all about how something interacts to affect the structure, composition, as well as properties of substances.
(c)
Answer to Problem 59A
The element with the chemical symbol and atomic number and mass number is:
Explanation of Solution
Protons are defined as positively charged
Electrons are negatively charged subatomic particles found orbiting the nucleus of an atom.
For neutral atom, the number of Electron = number of protons.
Neutrons are subatomic particles found in the nucleus of an atom which is neutral.
An atom has a nucleus with a positive charge due to its protons and has electrons in the space surrounding the nucleus at a relatively large distance from it.
For
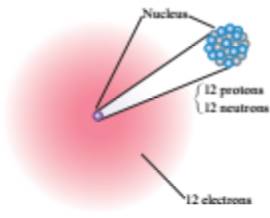
The element contains
The atomic number of the element is
And the mass number is
Hence the third element is
Chapter 3 Solutions
World of Chemistry, 3rd edition
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate please.arrow_forwardRelative Abundance 20- Problems 501 (b) The infrared spectrum has a medium-intensity peak at about 1650 cm. There is also a C-H out-of-plane bending peak near 880 cm. 100- 80- 56 41 69 M(84) LL 15 20 25 30 35 55 60 65 70 75 80 85 90 m/zarrow_forwardPolyethylene furanoate is a polymer made from plant-based sources; it is used for packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Phenol is the starting material for the synthesis of 2,3,4,5,6-pentachlorophenol, known al-ternatively as pentachlorophenol, or more simply as penta. At one time, penta was widely used as a wood preservative for decks, siding, and outdoor wood furniture. Draw the structural formula for pentachlorophenol and describe its synthesis from phenol.arrow_forward12 Mass Spectrometry (d) This unknown contains oxygen, but it does not show any significant infrared absorption peaks above 3000 cm . 59 100- BO 40 Relative Abundance M(102) - 15 20 25 30 35 40 45 50 5 60 65 70 75 80 85 90 95 100 105 mizarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: H HO H HO H HO H H -OH CH2OH Click and drag to start drawing a structure. Х : Darrow_forward
- : Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forwardDraw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forward
- Take a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forwardAnswer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





