
Concept explainers
In a blast furnace at high temperature, iron(III) oxide in ore reacts with carbon monoxide to produce metallic iron and carbon dioxide. The liquid iron produced is cooled and weighed. The reaction is run repeatedly with the same initial mass of iron(III) oxide, 19.0 g, but differing initial masses of carbon monoxide. The masses of iron obtained arc shown in this graph.
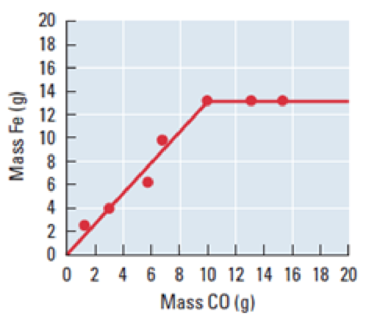
- (a) Write the balanced chemical equation for this reaction.
- (b) Calculate the mass of CO required to react completely with 19.0 g iron(III) oxide.
- (c) Calculate the mass of carbon dioxide produced when the reaction converts 10.0 g iron(III) oxide completely to products.
- (d) From the graph, determine which reactant is limiting when less than 10.0 g carbon monoxide is available to react with 19.0 g iron(III) oxide.
- (e) From the graph, determine which reactant is limiting when more than 10.0 g carbon monoxide is available to react with 19.0 g iron(III) oxide.
- (f) Calculate the percent yield if 24.0 g iron(III) oxide reacted with 20.0 g carbon monoxide to produce 15.9 g metallic iron.
- (g) Calculate the minimum mass of additional limiting reactant required to react with all of the excess of nonlimiting reactant from part (f).
(a)
Interpretation:
Iron
For the given reaction, balanced equation has to be written.
Concept introduction:
Balanced Chemical equation:
A balanced chemical equation is an equation which contains same elements in same number on both the sides (reactant and product side) of the chemical equation thereby obeying the law of conservation of mass.
Balancing the equation:
- There is a Law for conversion of mass in a chemical reaction i.e., the mass of total amount of the product should be equal to the total mass of the reactants.
- First write the skeletal reaction from the given information.
- Then count the number of atoms of each element in reactants as well as products.
- Place suitable coefficients in front of reactants as well as products until the number of atoms on each side (reactants and products) becomes equal.
Explanation of Solution
Given,
While balancing the equation, the subscripts cannot be altered but coefficients can be changed. There are two iron atoms in the left side and one iron atom in the right side of the reaction. Therefore, two iron atoms are added to right side of reaction. Now, the balanced equation is given below.
Three molecule of carbon monoxide is added to the left side of the reaction and three molecules of carbon dioxide is added to the right side of the reaction. Now, the balanced equation is given below.
(b)
Interpretation:
Mass of
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Therefore, mass of
Mass of
(c)
Interpretation:
Mass of carbon dioxide produced when the reaction converts
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Therefore, mass of
Mass of
(d)
Interpretation:
When less than
Concept introduction:
A limiting reactant is a reactant that is completely converted to products. Once all the limiting reactant is converted to products there is no other reactant to react.
Explanation of Solution
Given,
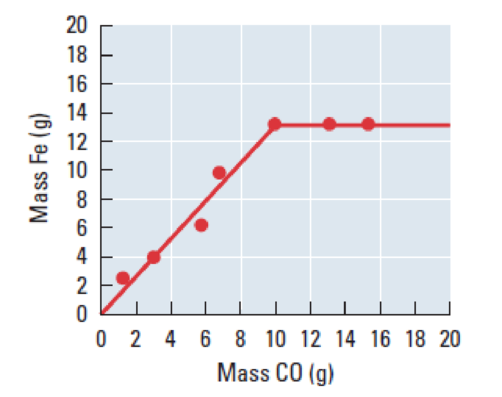
Figure 1
One mole of
The given graph between the mass of Fe and mass of carbon monoxide. The graph tells that the weight of the carbon monoxide is below
The calculation, shows that
(e)
Interpretation:
When more than
Concept introduction:
Refer to part (d)
Explanation of Solution
Given,
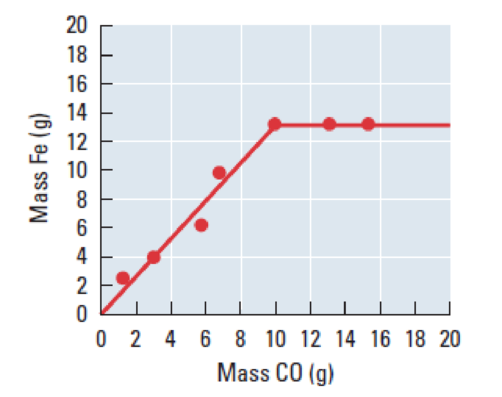
Figure 1
One mole of
The given graph between the mass of Fe and mass of carbon monoxide. The graph tells that the weight of the carbon monoxide is below
The graph tells that the weight of the carbon monoxide is more than
The calculation,
(f)
Interpretation:
When 24.0 g iron
Concept introduction:
The percent yield can be calculated by using following formula
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Given,
Number of moles of iron is calculated as follows,
Number of moles of iron is calculated as follows,
According to the mole calculation,
Amount of
The percent yield can be calculated by using following formula
The percent yield is
(g)
Interpretation:
The minimum mass of additional limiting reactant necessary to react with all of the excess of non-limiting reactant has to be calculated.
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Given,
Number of moles of iron is calculated as follows,
Number of moles of iron is calculated as follows,
According to the mole calculation,
Gram of
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: The Molecular Science
- For each of the Followin, moleaks draw all OF The Resonance contributing stuluctures and compare these three molecules in terms of Resonance stabilization 1-C-1 a. b. H A-C+ О 112-1 C. F-C-F Farrow_forwarda. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forwardC. Ν Harrow_forward
- a. H3C. N H3C CH3 HCNarrow_forwardол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forward
- At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





