
Concept explainers
(a)
Interpretation:
Based on the reaction, the product has to be predicted, name and formula for the product has to be given.
(a)
Explanation of Solution
Given,
Three Group 2A elements magnesium, calcium, and strontium are allowed to react with liquid bromine.
The product, name and formula for the product of the reaction is given below,
The magnesium metal reaction with bromine produces magnesium bromide
The calcium metal reaction with bromine produces calcium bromide
The strontium metal reaction with bromine produces strontium bromide
(b)
Interpretation:
The balanced equation has to be written for the given reaction.
Concept introduction:
Balanced Chemical equation:
A balanced chemical equation is an equation which contains same elements in same number on both the sides (reactant and product side) of the chemical equation thereby obeying the law of conservation of mass.
Balancing the equation:
- There is a Law for conversion of mass in a
chemical reaction i.e., the mass of total amount of the product should be equal to the total mass of the reactants. - First write the skeletal reaction from the given information.
- Then count the number of atoms of each element in reactants as well as products.
- Place suitable coefficients in front of reactants as well as products until the number of atoms on each side (reactants and products) becomes equal.
(b)
Explanation of Solution
Given,
Three Group 2A elements magnesium, calcium, and strontium are allowed to react with liquid bromine.
The balanced equation is given below,
The magnesium metal reaction with bromine produces magnesium bromide
The calcium metal reaction with bromine produces calcium bromide
The strontium metal reaction with bromine produces strontium bromide
(c)
Interpretation:
Nature of the reaction (acid-base reaction, precipitation reaction, or an oxidation-reduction reaction) has to be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the reactant combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
(c)
Explanation of Solution
Given,
Three Group 2A elements magnesium, calcium, and strontium are allowed to react with liquid bromine.
The magnesium metal reaction with bromine produces magnesium bromide
The given reaction is precipitation reaction, because the formation of
The calcium metal reaction with bromine produces calcium bromide
The given reaction is precipitation reaction, because the formation of
The strontium metal reaction with bromine produces strontium bromide
The given reaction is precipitation reaction, because the formation of
(d)
Interpretation:
Formula of each product has to be predicted.
(d)
Explanation of Solution
Given,
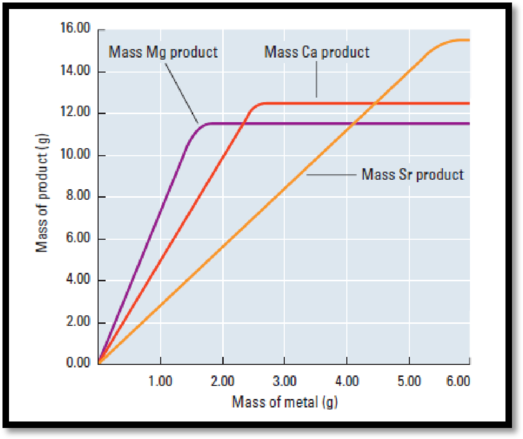
Figure 1
The mass of the metal is directly proportional to the mass of the compound formed.
From the graph, mass of sample is on the y-axis and the mass of the metal is on the x-axis and the fixed mass of the other reactant.
According to the graph (figure 1), mass of the compound is 11.4 g
Mass of the Mg (metal) is 1.6 g.
Consider the reaction takes place in 100 %. Then the mass of bromine is calculated as follows,
The molar mass of Mg is 24.3050 g/mole, the molar mass of bromine is 79.904 g/mole.
Number of moles of Mg is calculated below,
Number of moles of bromine is calculated below,
Therefore,
The ratio of the number of moles of Mg and bromine is given below,
0.066: 0.123
Divide through by smallest number:
Therefore, the Empirical formula for the given compound is
According to the graph (figure 1), mass of the compound is 12.4 g
Mass of the Ca (metal) is 2.5 g.
Consider the reaction takes place in 100 %. Then the mass of bromine is calculated as follows,
The molar mass of Ca is 40.078 g/mole, the molar mass of bromine is 79.904 g/mole.
Number of moles of Ca is calculated below,
Number of moles of bromine is calculated below,
Therefore,
The ratio of the number of moles of Ca and bromine is given below,
0.062: 0.124
Divide through by smallest number:
Therefore, the Empirical formula for the given compound is
According to the graph (figure 1), mass of the compound is 15.3 g
Mass of the Sr (metal) is 5.5 g.
Consider the reaction takes place in 100 %. Then the mass of bromine is calculated as follows,
The molar mass of Sr is 87.62 g/mole, the molar mass of bromine is 79.904 g/mole.
Number of moles of Sr is calculated below,
Number of moles of bromine is calculated below,
Therefore,
The ratio of the number of moles of Sr and bromine is given below,
0.063: 0.123
Divide through by smallest number:
Therefore, the Empirical formula for the given compound is
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: The Molecular Science
- molecule 0= OH ☐ ☐ type of molecule (check all that apply) fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated ☐ polyunsaturated ☐ ☐ ☐ ☐ ☐ 010 0 0 0 0 0 0 ☐ ☐ ☐ ☐☐☐☐ U omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 OH OHarrow_forward'☐ : ☑ ด Suppose an alien life form has DNA just like human DNA remain the same.) - except that the alien DNA is made from deoxyarabinose instead of deoxyribose. (All other ingredients Draw the structure of a nucleotide containing thymine from which the alien DNA would be assembled. Note: be sure to draw the molecule as it would exist at physiological pH. Click and drag to start drawing a structure.arrow_forwardPredict the products of the following biochemical reaction: CH2 CH-O + 3 KOH CH2-0 In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the No reaction box under the drawing area. Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw the complete right-hand side of the equation, including stoichiometric coefficients. No reaction Click and drag to start drawing a structure. : 5 èarrow_forward
- Name 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forward
- Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





