
Concept explainers
It is observed that as temperature is increased, most protein molecules go from their defined, folded state into a random-coil, denatured state that exposes more hydrophobic surface area than is exposed in the folded state.
a. Given what you have learned so about
b. Sometimes, however, proteins as temperature is decreased. How might this be explained? (Hint: Consider Problem 8)
7. Assume that some protein molecule, in its folded native state, has one favored conformation. But when it is denatured, it becomes a “random coil,” with many possible conformations.
a. If we only consider the entropy for the protein, what must be the sign of
b. How will the contribution of
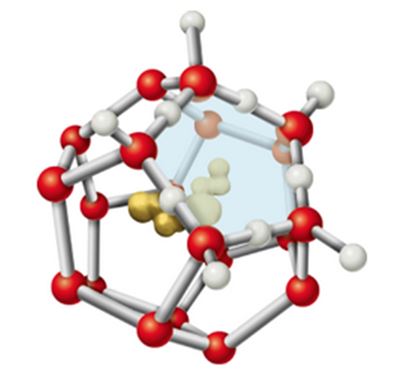
8. When a hydrophobic substance like a hydrocarbon is dissolved in water, a clathrate cage of ordered water molecules is formed about it (see Figure 2.14). If we consider only the effects on water, what do you expect the sign of

Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
- Show the fate of the proton on the 4-Oxygen molecule of F-1,6-BP. Please include a drawing showing the electron flow that occurs.arrow_forward1. Which one is the major organic product obtained from the following aldol condensation? O NaOH, H₂O heat A B C D Earrow_forwardAn organic chemist ordered the wrong item. She wanted to obtain 1-hydroxy-2-butanone, butinstead ordered 2-hydroxybutyraldehyde. As a good biochemist, show how the organic chemistcould use biological catalysis to make her desired compound. Please show the mechanism by drawing.arrow_forward
- Show the fate of the hydrogen on carbon-2 of glucose. Please draw out the structure using curve arrows to show electron flow.arrow_forward3. Which one of the compounds below is the major product formed by the reaction sequence shown here? CH3 + CH3NO2 NaOH H2, Ni ? nitromethane acetophenone OH OH HO HN- u x x x x Ph A HO -NH2 HO H Ph Ph Ph N- H B Ph NH2 D Earrow_forward4. Only ONE of the five compounds below can be prepared by an aldol condensation in which a single carbonyl compound is treated with base. Which one is it? To solve this problem, reverse the aldol condensation that formed each of these molecules to find out what two molecules came together to make the products. The one in which the two molecules are identical is the answer. Ph Ph ཚིག གནས ག ནཱ ཀ ན ཀནཱ A Ph H B Ph Ph H D Ph. Ph Ph E Harrow_forward
- 5. Which one is the major organic product obtained from the following reaction sequence? First, equimolar amounts of cyclopentanone and LDA are mixed at -78°C. Then propionaldehyde (propanal) is added. Addition of aqueous acid completes the process. LDA, -78°C. 1. 2. H₂O* H A B H 0 D H H Earrow_forward2. Which one is the major organic product obtained from the following reaction? NaOH, H₂O heat A B C D Earrow_forwardCH3CH2CHO + propanal PhCH2CHO 2-phenylacetaldehyde mixture of four products NaOH 10. In the crossed aldol reaction of propanal and 2-phenylacetaldehyde shown above, a mixture of four products will be formed. Which ONE of the compounds below will NOT be formed in this crossed aldol reaction? OH Ph A H OH OH Ph H B OH OH H H H Ph Ph C Ph D Earrow_forward
- An organic chemist ordered the wrong item. She wanted to obtain 1-hydroxy-2-butanone, butinstead ordered 2-hydroxybutyraldehyde. As a good biochemist, show how the organic chemistcould use biological catalysis to make her desired compound.arrow_forwardPredict the products of aldolase catalyzing the reaction with acetone and (S)-3-hydroxybutyraldehyde.arrow_forwardA cancer patient undergoing chemotherapy is taking a protein kinase inhibitor drug. The patientis an avid marathon runner and does not want to miss his upcoming race. Is it a good idea forthis patient to attempt a marathon while on this medication? Explain why or why not.arrow_forward
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





