
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 3.57SP
Interpretation Introduction
Interpretation:
From the given electronic configurations a tripositive ion and a neutral atom hasto be identified.
Concept introduction:
By using aufbau principle, electrons starts to occupy from orbitals oflower energy level. Thus, the obtained lower energy configuration is known as ground-state electron configuration.
Aufbau principle:
- Electrons first occupy orbitals with lower energy than higher energy.
- An orbital can be occupied only by two electrons having opposite spins.
- Each electron fills each orbital till it is half filled, when they are degenerate orbital. This is called as Hund’s rule.
- The order of electrons filling in an atom is given as
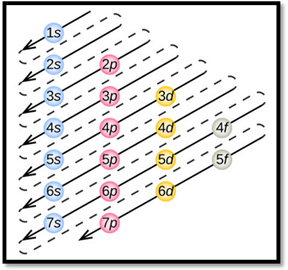
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Including activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4
Ksp Hg2Br2 = 5.6×10-23.
give example for the following(by equation)
a. Converting a water insoluble compound to a soluble one.
b. Diazotization reaction form diazonium salt
c. coupling reaction of a diazonium salt
d. indacator properties of MO
e. Diazotization ( diazonium salt of bromobenzene)
2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.
Chapter 3 Solutions
General Chemistry: Atoms First
Ch. 3.1 - Prob. 3.1PCh. 3.1 - Which of the following drawings is most likely to...Ch. 3.2 - Give systematic names for the following compounds:...Ch. 3.2 - Write formulas for the following compounds: (a)...Ch. 3.2 - Prob. 3.5CPCh. 3.2 - Give systematic names for the following compounds:...Ch. 3.2 - Prob. 3.7PCh. 3.2 - Prob. 3.8CPCh. 3.3 - Predict the ground-state electron configuration...Ch. 3.3 - What doubly positive ion has the following...
Ch. 3.4 - Prob. 3.11PCh. 3.4 - which of the following spheres represents a K+...Ch. 3.5 - Using the periodic table as your guide, predict...Ch. 3.6 - (a) Which has the larger third ionization energy,...Ch. 3.6 - Three atoms have the following electron...Ch. 3.6 - Order the indicated three elements according to...Ch. 3.7 - Prob. 3.17PCh. 3.7 - Which of the indicated three elements has the...Ch. 3.8 - What noble-gas configurations are the following...Ch. 3.8 - Prob. 3.20PCh. 3.9 - Calculate the net energy change in kilojoules per...Ch. 3.10 - Which substance in each of the following pairs has...Ch. 3.10 - One of the following pictures represents NaCl and...Ch. 3.11 - Prob. 3.24PCh. 3.11 - Complete the following equations so that the same...Ch. 3.12 - Prob. 3.26PCh. 3.12 - Prob. 3.27PCh. 3.14 - Prob. 3.28PCh. 3 - In the following drawings, red spheres represent...Ch. 3 - Which of the following drawings is more likely to...Ch. 3 - Prob. 3.31CPCh. 3 - Prob. 3.32CPCh. 3 - Prob. 3.33CPCh. 3 - Prob. 3.34CPCh. 3 - Prob. 3.35CPCh. 3 - Prob. 3.36CPCh. 3 - Prob. 3.37CPCh. 3 - Prob. 3.38SPCh. 3 - Prob. 3.39SPCh. 3 - Prob. 3.40SPCh. 3 - Prob. 3.41SPCh. 3 - Prob. 3.42SPCh. 3 - Prob. 3.43SPCh. 3 - Prob. 3.44SPCh. 3 - Prob. 3.45SPCh. 3 - Prob. 3.46SPCh. 3 - Prob. 3.47SPCh. 3 - Prob. 3.48SPCh. 3 - Prob. 3.49SPCh. 3 - Prob. 3.50SPCh. 3 - Prob. 3.51SPCh. 3 - Prob. 3.52SPCh. 3 - What is the identity of the element X in the...Ch. 3 - Prob. 3.54SPCh. 3 - Prob. 3.55SPCh. 3 - Prob. 3.56SPCh. 3 - Prob. 3.57SPCh. 3 - Prob. 3.58SPCh. 3 - Prob. 3.59SPCh. 3 - Prob. 3.60SPCh. 3 - Prob. 3.61SPCh. 3 - Prob. 3.62SPCh. 3 - Prob. 3.63SPCh. 3 - Prob. 3.64SPCh. 3 - Prob. 3.65SPCh. 3 - Prob. 3.66SPCh. 3 - Prob. 3.67SPCh. 3 - Which element in each of the following sets has...Ch. 3 - Prob. 3.69SPCh. 3 - Prob. 3.70SPCh. 3 - Prob. 3.71SPCh. 3 - Prob. 3.72SPCh. 3 - Prob. 3.73SPCh. 3 - Prob. 3.74SPCh. 3 - Prob. 3.75SPCh. 3 - Prob. 3.76SPCh. 3 - Prob. 3.77SPCh. 3 - Prob. 3.78SPCh. 3 - Order the following compounds according to their...Ch. 3 - Calculate the energy change in kilojoules per mole...Ch. 3 - Prob. 3.81SPCh. 3 - Prob. 3.82SPCh. 3 - Prob. 3.83SPCh. 3 - Prob. 3.84SPCh. 3 - Prob. 3.85SPCh. 3 - Calculate the overall energy change in kilojoules...Ch. 3 - The estimated lattice energy for CsF2(s) is +2347...Ch. 3 - Prob. 3.88SPCh. 3 - Prob. 3.89SPCh. 3 - Prob. 3.90SPCh. 3 - Prob. 3.91SPCh. 3 - Prob. 3.92SPCh. 3 - Prob. 3.93SPCh. 3 - Prob. 3.94SPCh. 3 - Prob. 3.95SPCh. 3 - Prob. 3.96SPCh. 3 - Prob. 3.97SPCh. 3 - Prob. 3.98SPCh. 3 - Prob. 3.99SPCh. 3 - Prob. 3.100CHPCh. 3 - Prob. 3.101CHPCh. 3 - Prob. 3.102CHPCh. 3 - Prob. 3.103CHPCh. 3 - Prob. 3.104CHPCh. 3 - Prob. 3.105CHPCh. 3 - Prob. 3.106CHPCh. 3 - Prob. 3.107CHPCh. 3 - Prob. 3.108CHPCh. 3 - Prob. 3.109CHPCh. 3 - Prob. 3.110CHPCh. 3 - Prob. 3.111CHPCh. 3 - Prob. 3.112CHPCh. 3 - Prob. 3.113CHPCh. 3 - Prob. 3.114CHPCh. 3 - Given the following information, construct a...Ch. 3 - Given the following information, construct a...Ch. 3 - Consider the electronic structure of the element...Ch. 3 - Prob. 3.118MPCh. 3 - Prob. 3.119MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forwardWhat is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forward
- A 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forwardwrite the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forward
- The compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forwardCaffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forward
- Indicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forwardWrite chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY