
Concept explainers
3-38 Complete the chart by writing formulas for the compounds formed:
| Br- | MnO4- | O2- | NO3- | SO42- | PO43- | OH- | |
| Li+ | |||||||
| Ca2+ | |||||||
| Co3+ | |||||||
| K+ | |||||||
| Cu2+ |
Interpretation:
Complete the chart by writing formulas.
Concept Introduction:
The compounds which contain ionic bond, which is a type of bond which formed between positive metal ion and negative, non-metal ions.
Answer to Problem 3.38P
The complete chart is as follows:
Explanation of Solution
Polyatomic ion is a group of covalently bonded nonmetal atoms which has an overall electrical charge.
In general, there are two types of ions: positive metal ions and negative non −metal ions.
When a neutral element gain electrons, the negative charge increases on it and it convert into negative ion. This negative ion is always greater than its parent atom.
Similarly, when a neutral element loss electrons, the negative charge decreases on it and it convert into positive ion. This positive ion is always smaller than its parent atom.
The general rules for writing formula of binary compound are as follows:
- To write the formula of positive ion first.
- The write the formula of negative ions after positive ion.
- Using criss-cross method, the charges of both the ions are interchanged.
- The formula shows the lowest whole-number ratio of both the ions.
For example: the symbols of the magnesium and chloride ions are as follows:
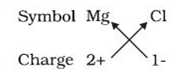
The formula of the ionic compound of these ions is as follows:
The complete chart is as follows:
The chart was completed using the criss-cross method, in which the respective ions are written with their formulas and charge and are then cross multiplied.
Want to see more full solutions like this?
Chapter 3 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
