
Concept explainers
(a)
Interpretation:
The number of
Concept introduction:
In order to determine the number of
In the Lewis structure, a single bond represents an electron pair in a
Answer to Problem 3.18P
There are sixteen
Explanation of Solution
The line drawing of the given molecule is:
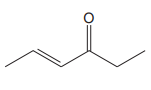
This must be converted to a Lewis structure showing all atoms and lone pairs before the number of bonds of different types and the electrons in nonbonding MOs can be counted.
The Lewis structure of the molecule showing all atoms, bonds and lone pairs can be drawn as:
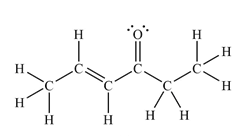
This shows fourteen single bonds, two double bonds, and two lone pairs. Therefore, the molecule contains a total of sixteen
A single bond between two atoms is a
(b)
Interpretation:
The number of
Concept introduction:
In order to determine the number of
In the Lewis structure, a single bond represents an electron pair in a
Answer to Problem 3.18P
There are sixteen
Explanation of Solution
The line drawing of the given molecule is:
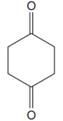
This must be converted to a Lewis structure showing all atoms and lone pairs before the number of bonds of different types and the electrons in nonbonding MOs can be counted.
The Lewis structure of the molecule showing all atoms, bonds and lone pairs can be drawn as:
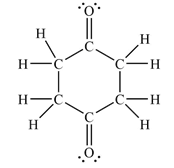
This shows fourteen single bonds, two double bonds, and four lone pairs. Therefore, the molecule contains a total of sixteen
A single bond between two atoms is a
(c)
Interpretation:
The number of
Concept introduction:
In order to determine the number of
In the Lewis structure, a single bond represents an electron pair in a
Answer to Problem 3.18P
There are twelve
Explanation of Solution
The line drawing of the given molecule is:
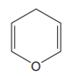
This must be converted to a Lewis structure showing all atoms and lone pairs before the number of bonds of different types and the electrons in nonbonding MOs can be counted.
The Lewis structure of the molecule showing all atoms, bonds and lone pairs can be drawn as:
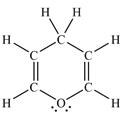
This shows twelve single bonds, two double bonds, and two lone pairs. Therefore, the molecule contains a total of twelve
A single bond between two atoms is a
(d)
Interpretation:
The number of
Concept introduction:
In order to determine the number of
In the Lewis structure, a single bond represents an electron pair in a
Answer to Problem 3.18P
There are nineteen
Explanation of Solution
The line drawing of the given molecule is:
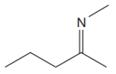
This must be converted to a Lewis structure showing all atoms and lone pairs before the number of bonds of different types and the electrons in nonbonding MOs can be counted.
The Lewis structure of the molecule showing all atoms, bonds and lone pairs can be drawn as:
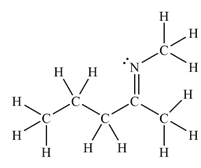
This shows eighteen single bonds, one double bond and one lone pair. Therefore, the molecule contains a total of nineteen
A single bond between two atoms is a
(e)
Interpretation:
The number of
Concept introduction:
In order to determine the number of
In the Lewis structure, a single bond represents an electron pair in a
Answer to Problem 3.18P
There are eleven
Explanation of Solution
The line drawing of the given molecule is:
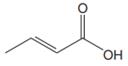
This must be converted to a Lewis structure showing all atoms and lone pairs before the number of bonds of different types and the electrons in nonbonding MOs can be counted.
The Lewis structure of the molecule showing all atoms, bonds and lone pairs can be drawn as:
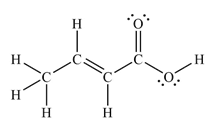
This shows nine single bonds, two double bonds, and four lone pairs. Therefore, the molecule contains a total of eleven
A single bond between two atoms is a
Want to see more full solutions like this?
Chapter 3 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- If I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forward
- If I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained when acetophenone reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of N-(4-methylphenyl)acetamide with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 4-(trifluoromethyl)benzonitrile with a sulfonitric mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
