
Concept explainers
(a)
Interpretation:
One alternative tautomeric structure for Adenine has to be written.
Concept Introduction:
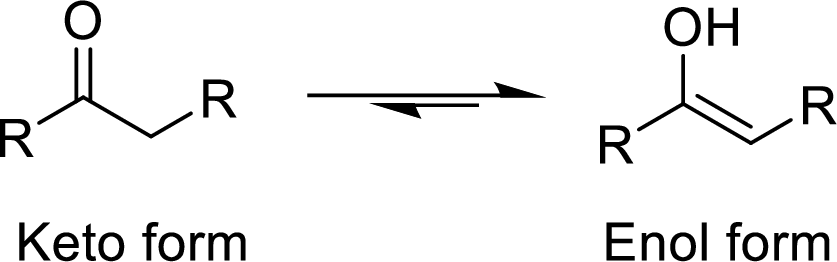
The isomers which differ in the position of the protons and electrons and the carbon skeleton is same are said to be Tautomers and the mechanism is known as Tautomerism. The common example for Tautomerism is keto-enol tautomerization. The keto group is converted to enol (alcohol) group by the intramolecular exchange of protons.
(b)
Interpretation:
Will the tautomeric structure of Adenine base pairs with Thymine or any other base has to be given.
Concept Introduction:
In the DNA double helix, the bases are paired with each other on the adjacent strands to hold the double helix. The bases present in DNA are Adenine, Guanine, Cytosine and Thymine. The pairing occurs between the Adenine and Thymine (A-T) and Guanine and Cytosine (G-C).
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





