
Concept explainers
(a)
Interpretation:
The explanation for alanine which has different optical rotation in water,
Concept introduction:
The amino acid is made of two
Answer to Problem 27.61AP
Each solvent rotates the alanine molecule to a different angle due to the formation of different complex formation and so it gives rise to different optical rotation in different solvents.
Explanation of Solution
The structure of alanine is shown below.
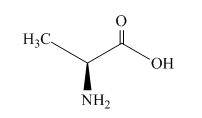
Figure 1
Alanine is an optically active compound. It rotates the plane of polarized light. The reaction of alanine with water,
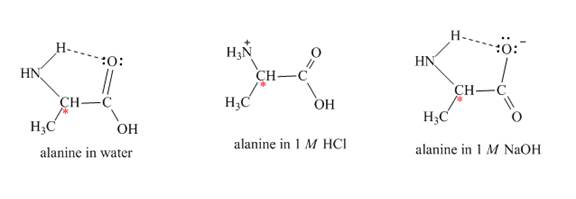
Figure 2
The optical rotation of alanine is measure by Circular Dichroism (CD). It involves circularly polarized light absorption. It measures the angle at which the plane-polarized light is rotated by the molecule. Each solvent rotates the alanine molecule to a different angle, due to this it gives rise to different optical rotation in different solvents.
The alanine has different optical rotation in water,
(b)
Interpretation:
The explanation for two known mono
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
Due to the presence of two amine groups in lysine molecule. It may undergo acetylation reaction from either side and form mono
Explanation of Solution
The structure of amino acid lysine is shown below.
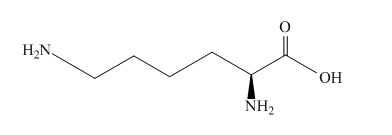
Figure 3
The structure of the lysine molecule contains two amine group one at the
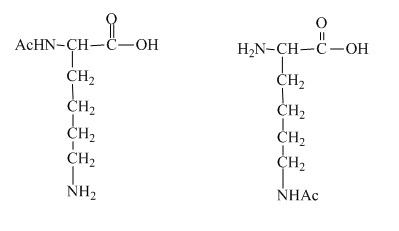
Figure 4
The two mono
(c)
Interpretation:
The explanation for fact that the peptide
Concept introduction:
The amino acid is made of two functional groups an amine group
Answer to Problem 27.61AP
The compound urea under basic conditions acts as a denaturation agent which breakdown the protein molecule bonding. Due to this, the peptide,
Explanation of Solution
The protein molecule is composed of four types of structure primary, secondary, tertiary and quarternary. The enzyme trypsin hydrolyzes the peptide,
The peptide
(d)
Interpretation:
The explanation for the peptide containing cysteine on reaction with
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
The generation of lysine type molecule at the end of the reaction which is cleaved by trypsin enzyme. Due to this, the peptide containing cysteine on reaction with
Explanation of Solution
The structure of the cysteine molecule is shown below.
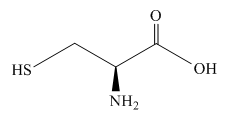
Figure 5
The same molecule in peptides exists as a disulfide bond. The disulfide bond of cysteine in the protein molecule is shown below.
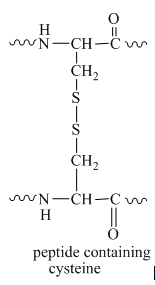
Figure 6
When this protein molecule with a disulfide bond is treated with
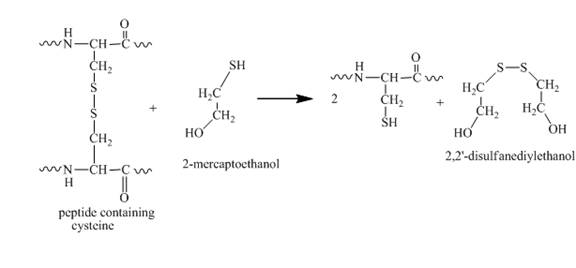
Figure 7
The thiol group is then reacted with the aziridine molecule which results in the formation of a lysine type molecule. This reaction is shown below.
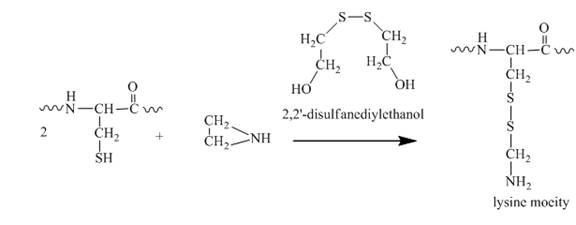
Figure 8
This lysine type molecule then reacts with trypsin enzyme which cleaves arginine and lysine molecule. Due to this, the trypsin enzyme reacts with the modified cysteine residues.
The peptide containing cysteine on reaction with
(e)
Interpretation:
The explanation for the formation of two separable methionine sulfoxides from the oxidation of
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
The application of a certain amount of energy which converts one form to another form of structure. Due to this, the formation of two separable methionine sulfoxides from the oxidation of
Explanation of Solution
The structure of methionine is shown below.
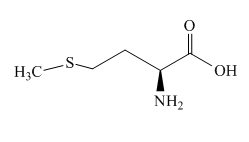
Figure 9
The resonance structure of sulfoxides with two different groups is shown below.
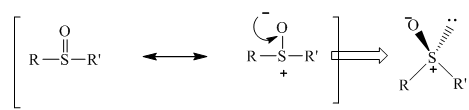
Figure 10
The conversion of one structure to another structure at room temperature requires a certain amount of energy. Therefore, on the application of that amount of energy, the two forms of
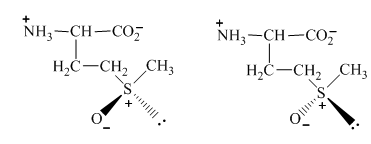
Figure 11
The formation of two separable methionine sulfoxides from the oxidation of
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry Study Guide and Solutions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

