
(a)
Interpretation:
The structure of the product expected when valine would react with
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three-word abbreviation or one-word abbreviation is used for amino acids.
Answer to Problem 27.43AP
The structure of the product expected when valine would react with
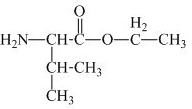
The structure of the product expected when proline would react with
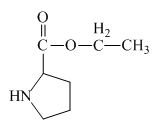
Explanation of Solution
The valine has a carboxylic group in it. This carboxylic group can undergo esterification with ethanol in the presence of sulfuric acid. The corresponding
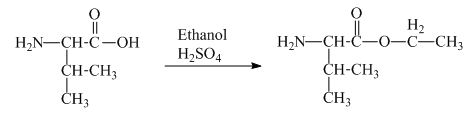
Figure 1
The proline has a carboxylic group in it. This carboxylic group can undergo esterification with ethanol in the presence of sulfuric acid. The corresponding chemical reaction is shown below.
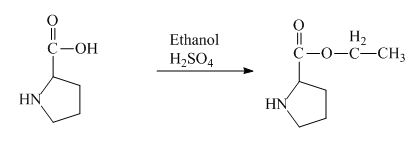
Figure 2
The structure of the product expected when valine would react with
(b)
Interpretation:
The structure of the product expected when valine would react with benzoyl chloride and
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three-word abbreviation or one-word abbreviation is used for amino acids.
Answer to Problem 27.43AP
The structure of the product expected when valine would react with benzoyl chloride and ![]() is shown below.
is shown below.
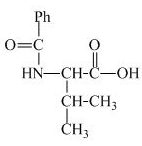
The structure of the product expected when proline would react with benzoyl chloride and
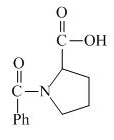
Explanation of Solution
The valine has a basic
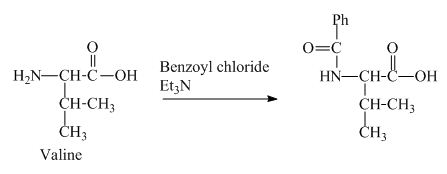
Figure 3
The proline has a basic amine group in it. This group can react with benzoyl chloride to form amide bond. The corresponding chemical reaction is shown below.
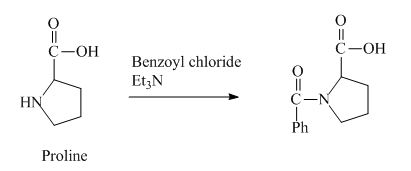
Figure 4
The structure of the product expected when valine would react with benzoyl chloride and
The structure of the product expected when proline would react with benzoyl chloride and
(c)
Interpretation:
The structure of the product expected when valine would react with an aqueous
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three-word abbreviation or one-word abbreviation is used for amino acids.
Answer to Problem 27.43AP
The structure of the product expected when valine would react with an aqueous
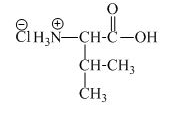
The structure of the product expected when proline would react with an aqueous
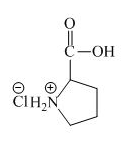
Explanation of Solution
The valine has a basic amine group in it. This amine group can react with acid to form an ammonium base. The corresponding chemical reaction is shown below.
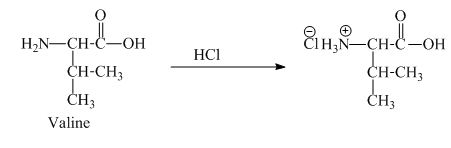
Figure 5
The proline has a basic amine group in it. This amine group can react with acid to form an ammonium base. The corresponding chemical reaction is shown below.
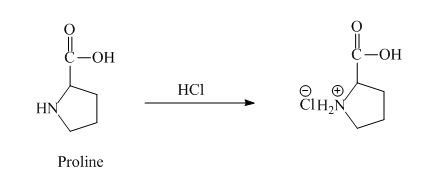
Figure 6
The structure of the product expected when valine would react with an aqueous
The structure of the product expected when proline would react with an aqueous
(d)
Interpretation:
The structure of the product expected when valine would react with an aqueous
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three-word abbreviation or one-word abbreviation is used for amino acids.
Answer to Problem 27.43AP
The structure of the product expected when valine would react with an aqueous
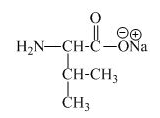
The structure of the product expected when proline would react with an aqueous
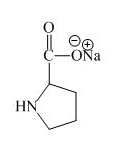
Explanation of Solution
The valine has an acidic carboxylic group in it. This group can react with a base such as sodium hydroxide to form a sodium salt of
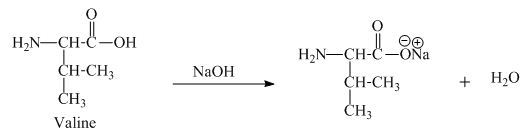
Figure 7
The proline has an acidic carboxylic group in it. This group can react with a base such as sodium hydroxide to form a sodium salt of carboxylic acid. The corresponding chemical reaction is shown below.
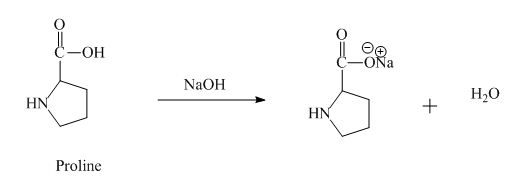
Figure 8
The structure of the product expected when valine would react with an aqueous
The structure of the product expected when proline would react with an aqueous
(e)
Interpretation:
The structure of the product expected when valine would react with aqueous benzaldehyde and
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three-word abbreviation or one-word abbreviation is used for amino acids.
Answer to Problem 27.43AP
The structure of the product expected when valine would react with aqueous benzaldehyde and
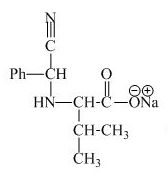
The structure of the product expected when proline would react with aqueous benzaldehyde and
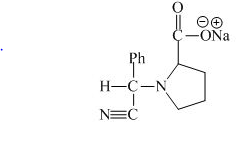
Explanation of Solution
The valine has a basic group in it. This group can be attacked by the benzaldehyde in the presence of sodium cyanide to form the final product. The corresponding chemical reaction is shown below.
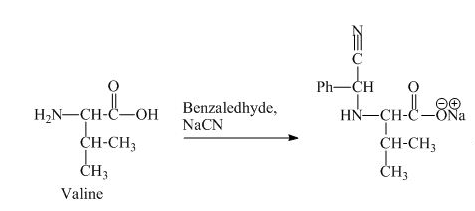
Figure 9
The proline has a basic group in it. This group can be attacked by the benzaldehyde in the presence of sodium cyanide to form the final product. The corresponding chemical reaction is shown below.
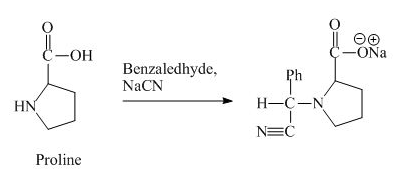
Figure 10
The structure of the product expected when valine would react with aqueous benzaldehyde and
The structure of the product expected when proline would react with aqueous benzaldehyde and
(f)
Interpretation:
The structure of the product expected when valine would react with aqueous Fmoc-![]() followed by neutralizing with
followed by neutralizing with
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three-word abbreviation or one-word abbreviation is used for amino acids.
Answer to Problem 27.43AP
The structure of the product expected when valine would react with aqueous Fmoc-
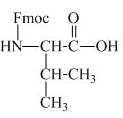
The structure of the product expected when proline would react with aqueous Fmoc-
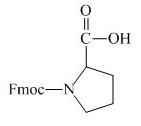
Explanation of Solution
The valine has an amine group. This group can be protected by the reaction of valine with aqueous Fmoc-
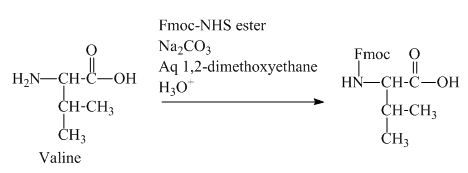
Figure 11
The proline has an amine group. This group can be protected by the reaction of proline with aqueous Fmoc-
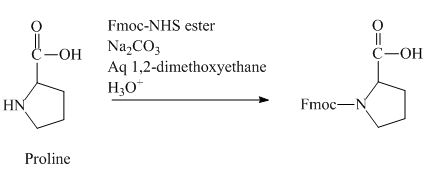
Figure 12
The structure of the product expected when valine would react with aqueous Fmoc-
The structure of the product expected when proline would react with aqueous Fmoc-
(g)
Interpretation:
The structure of the product expected when the given derivative of valine would react with ![]() butyl ester is to be predicted. The structure of the product expected when the given derivative of proline would react with
butyl ester is to be predicted. The structure of the product expected when the given derivative of proline would react with ![]() butyl ester is to be predicted.
butyl ester is to be predicted.
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. These
Answer to Problem 27.43AP
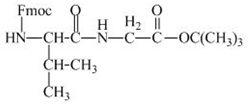
The structure of the product expected when the given derivative of valine would react with ![]() butyl ester is shown below.
butyl ester is shown below.
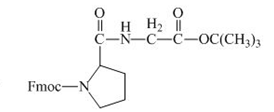
The structure of the product expected when the given derivative of proline would react with ![]() butyl ester is below.
butyl ester is below.
Explanation of Solution
The valine has an amine group. This group can be protected by the reaction of valine with aqueous Fmoc-![]() butyl ester. The corresponding chemical reaction is shown below.
butyl ester. The corresponding chemical reaction is shown below.
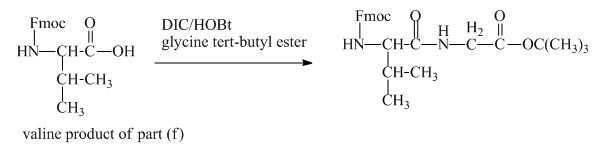
Figure 13
The carboxylic group of the derivative of proline can be protected with the help of reagent with ![]() butyl ester. The corresponding chemical reaction is shown below.
butyl ester. The corresponding chemical reaction is shown below.
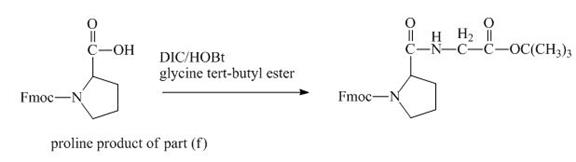
Figure 14
The structure of the product expected when the given derivative of valine would react with ![]() butyl ester is shown in Figure 13.
butyl ester is shown in Figure 13.
The structure of the product expected when the given derivative of proline would react with ![]() butyl ester is shown in Figure 14.
butyl ester is shown in Figure 14.
(h)
Interpretation:
The structure of the product expected when the given derivative of valine would react with anhydrous
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. These functional groups can be protected by different reagent to synthesize peptide chains. The protecting groups can be removed by hydrolysis.
Answer to Problem 27.43AP
The structure of the product expected when the given derivative of valine would react with anhydrous
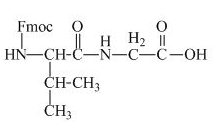
The structure of the product expected when the given derivative of proline would react with anhydrous
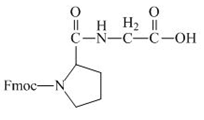
Explanation of Solution
The derivative of valine has an ester linkage in it. The acid,
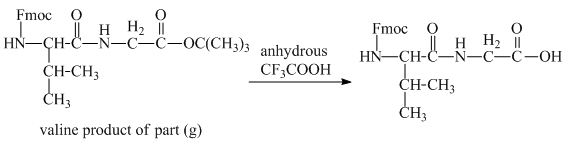
Figure 15
The derivative of proline has an ester linkage in it. The acid,
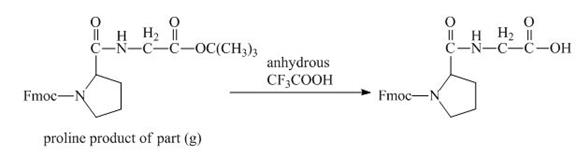
Figure 16
The structure of the product expected when the given derivative of valine would react with anhydrous
The structure of the product expected when the given derivative of proline would react with anhydrous
(i)
Interpretation:
The structure of the product expected when the given derivative of valine would react with
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. These functional groups can be protected by different reagent to synthesize peptide chains. The protecting groups can be removed by hydrolysis.
Answer to Problem 27.43AP
The structure of the product expected when the given derivative of valine would react with
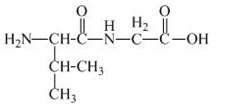
The structure of the product expected when the given derivative of proline would react with
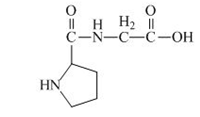
Explanation of Solution
The solution of
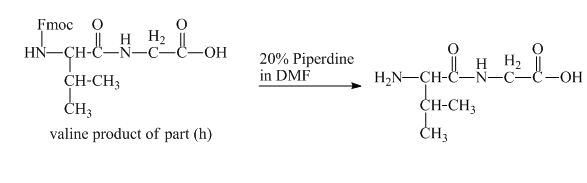
Figure 17
The solution of
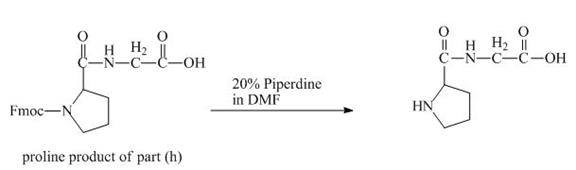
Figure 18
The structure of the product expected when the given derivative of valine would react with
The structure of the product expected when the given derivative of proline would react with
(j)
Interpretation:
The structure of the product expected when the given derivative of valine would react with
Concept introduction:
The chemical compounds in which carbon is bonded with the acidic and basic group along with hydrocarbon side chain are known as amino acids. These functional groups can be protected by different reagent to synthesize peptide chains. The protecting groups can be removed by hydrolysis.
Answer to Problem 27.43AP
The structure of the product expected when the given derivative of valine would react with
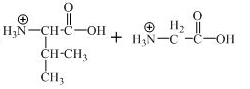
The structure of the product expected when the given derivative of proline would react with
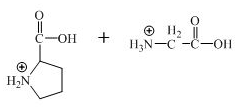
Explanation of Solution
The derivative of valine has amide linkage in it. This amide linkage can be hydrolysis by heating with a strong acid such as
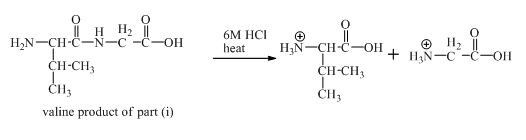
Figure 19
The derivative of valine has amide linkage in it. This amide linkage can be hydrolysis by heating with a strong acid such as
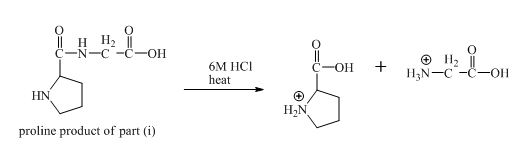
Figure 20
The structure of the product expected when the given derivative of valine would react with
The structure of the product expected when the given derivative of proline would react with
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry Study Guide and Solutions
- Answe Answer A and B pleasearrow_forward3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forward
- Leucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai 2.36 and pKa2 = 9.60. H2N-C(R)H-COOH and R is -CH2-CH(CH3)2 A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk. B. How many possible stereoisomers of leucine are there? C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S. D. What is the p/ of leucine? E. Draw the structure of the predominant form of leucine at 10.00. F. Draw the structure of the predominant form of leucine at pH = 1.50. G. Leucine is described as an essential amino acid. What does this mean? H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method. =arrow_forwarda) Write out 6 completely different reactions of acetophenone (reagent, product). b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain. c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain.arrow_forward12. CH3 OH OH H&C CH3 H₂C N OH H₂C CH3 H&C CH3 H₂C' CH3 H.C CH3OH H.C CH2CH3OH CH3CEN Which one of these 17 compounds is represented by this IR and this 'H NMR spectrum? IR Spectrum 3000 4000 3000 NMR Spectrum 2000 £500 RAVENUMBER 2000 1500 9 8 6 5 10 HP-00-290 ppm m 1000 500 1000 4 °arrow_forward
- Draw the structure of (E,6R) 6-methoxy-4-hepten-2-one. Give the IUPAC name of this compound, including stereochemistry. Draw the most stable chair conformation of (cis) 1,3-isobutylcyclohexane. H HC=CCH₂ CH2CH3 EN(CH3)2 -CN(CH3)2arrow_forward10. Write out the mechanism (intermediate/transition state) for this reaction; indicate stereochemistry in product. H3C CH₂OH CH3 SN1 Harrow_forwardWrite "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forward
- Write "most" under the most acidic compound. Write "least" under the least acidic compound. OH NO₂ OCH3 Br 9. Compound X, C50H84F2, reacts with excess H2/Pd to give a C50H88F2 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forward4. State whether these two are: a) the same molecule b) c) d) different compounds that are not isomers constitutional isomers diastereomers e) enantiomers CH3 CH₁₂ H OH HO H H OH HO H CH, CH₂ 5. a) How many stereocenters does this compound have? b) How many stereoisomers are possible for this compound? CH₂ OH CHCHarrow_forwardCalculating the pH at equivalence of a titration A chemist titrates 210.0 mL of a 0.1003 M hydrobromic acid (HBr) solution with 0.7550M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = ] ☑ o0o 18 Ararrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


