
Concept explainers
(a)
Interpretation:
The pairing of bile and solubilizing agent is correct or not has to be determined.
Concept introduction:
Bile is a yellowish-green fluid which is released from the liver and is used for digesting and absorbing fats and their soluble vitamins in the small intestine which cause an increment of absorption of fats.
(b)
Interpretation:
The pairing of chylomicron and monoacylglycerol formation is correct or not has to be determined.
Concept introduction:
Triacylglycerols are lipid molecules that are formed by fatty acids. They constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the
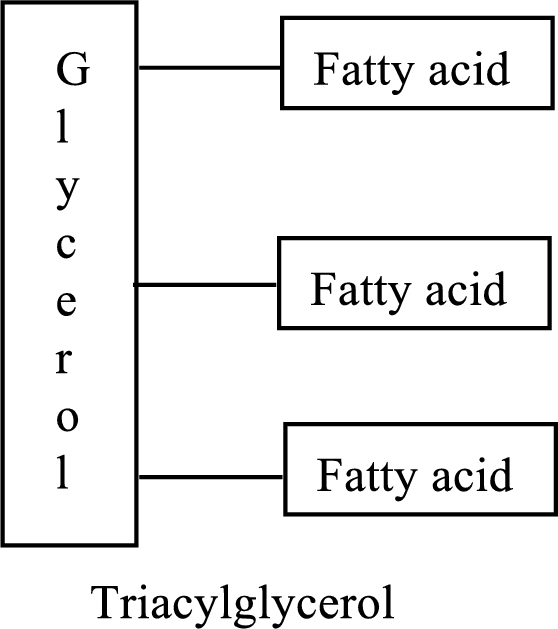
(c)
Interpretation:
The pairing of cholecystokinin and bile release is correct or not has to be determined.
Concept introduction:
Triacylglycerols are lipid molecules that are formed by fatty acids. They constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the digestive system and are later absorbed into the bloodstream. The structure of triacylglycerols is as follows:
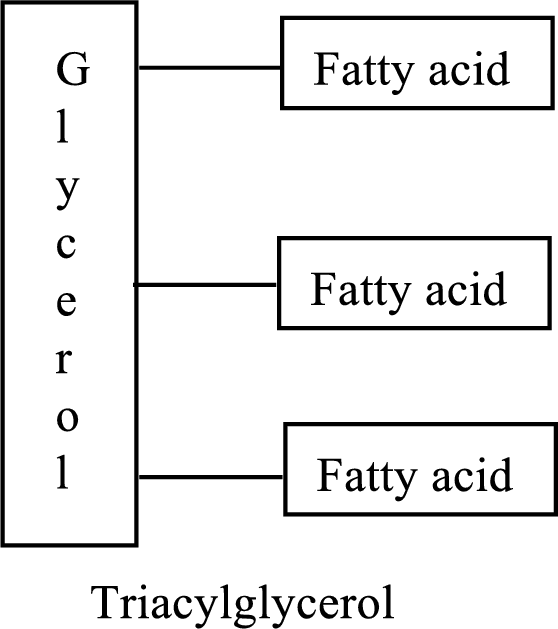
Bile is a yellowish-green fluid which is released from the liver and is used for digesting and absorbing fats and their soluble vitamins in the small intestine which cause an increment of absorption of fats.
(d)
Interpretation:
The pairing of chyme and partially digested food is correct or not has to be determined.
Concept introduction:
Triacylglycerols are lipid molecules that are formed by fatty acids. They constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the digestive system and are later absorbed into the bloodstream. The structure of triacylglycerols is as follows:
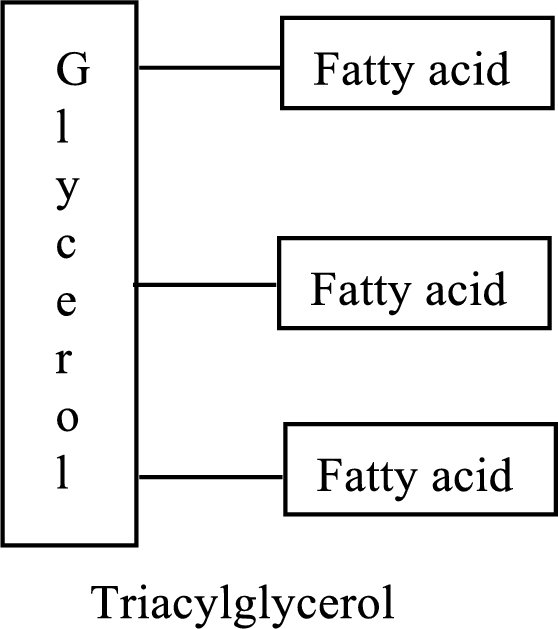
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- The following table is from Kumar et. al. Highly Selective Dopamine D3 Receptor (DR) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment. J. Med Chem 2016.arrow_forwardThe following figure is from Caterina et al. The capsaicin receptor: a heat activated ion channel in the pain pathway. Nature, 1997. Black boxes indicate capsaicin, white circles indicate resinferatoxin. You are a chef in a fancy new science-themed restaurant. You have a recipe that calls for 1 teaspoon of resinferatoxin, but you feel uncomfortable serving foods with "toxins" in them. How much capsaicin could you substitute instead?arrow_forwardWhat protein is necessary for packaging acetylcholine into synaptic vesicles?arrow_forward
- 1. Match each vocabulary term to its best descriptor A. affinity B. efficacy C. inert D. mimic E. how drugs move through body F. how drugs bind Kd Bmax Agonist Antagonist Pharmacokinetics Pharmacodynamicsarrow_forward50 mg dose of a drug is given orally to a patient. The bioavailability of the drug is 0.2. What is the volume of distribution of the drug if the plasma concentration is 1 mg/L? Be sure to provide units.arrow_forwardDetermine Kd and Bmax from the following Scatchard plot. Make sure to include units.arrow_forward
- Choose a catecholamine neurotransmitter and describe/draw the components of the synapse important for its signaling including synthesis, packaging into vesicles, receptors, transporters/degradative enzymes. Describe 2 drugs that can act on this system.arrow_forwardThe following figure is from Caterina et al. The capsaicin receptor: a heat activated ion channel in the pain pathway. Nature, 1997. Black boxes indicate capsaicin, white circles indicate resinferatoxin. a) Which has a higher potency? b) Which is has a higher efficacy? c) What is the approximate Kd of capsaicin in uM? (you can round to the nearest power of 10)arrow_forwardWhat is the rate-limiting-step for serotonin synthesis?arrow_forward
 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage





