
General, Organic, and Biological Chemistry Seventh Edition
7th Edition
ISBN: 9781305767867
Author: H. Stephan Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 25.4, Problem 5QQ
Interpretation Introduction
Interpretation:
The number of turns of the β-oxidation pathway needed to “process” a C16 saturated fatty acid has to be determined.
Concept introduction:
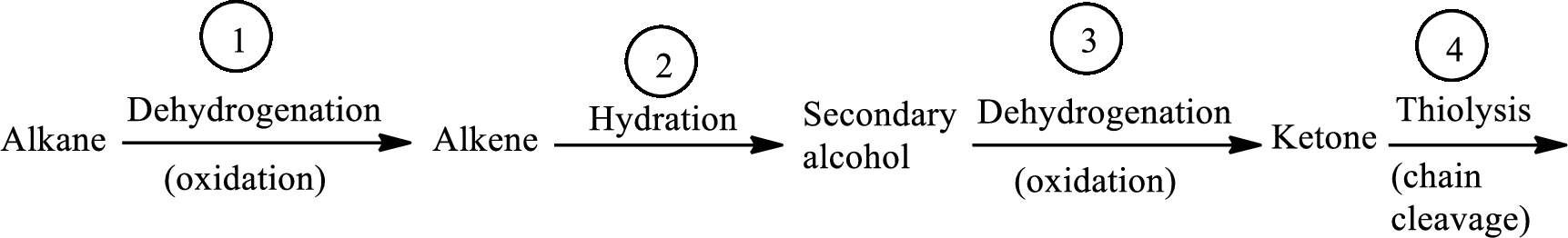
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway. The reaction types and the
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the stepwise mechanism for the reactions
Part I.
a)
Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone
b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone
(3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism
the formation of
the products
For
3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below:
Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life).
2
CH3
H
NO2
NO2
3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s)
H
a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.
Chapter 25 Solutions
General, Organic, and Biological Chemistry Seventh Edition
Ch. 25.1 - Which of the following statements about digestion...Ch. 25.1 - Prob. 2QQCh. 25.1 - The major function of bile released during...Ch. 25.1 - The two major products of triacylglycerol...Ch. 25.1 - Prob. 5QQCh. 25.2 - Hormone-sensitive lipase needed for...Ch. 25.2 - Prob. 2QQCh. 25.2 - Which of the following is not a product of...Ch. 25.3 - Prob. 1QQCh. 25.3 - What is the intermediate compound in the two-step...
Ch. 25.3 - Prob. 3QQCh. 25.4 - Prob. 1QQCh. 25.4 - Prob. 2QQCh. 25.4 - Prob. 3QQCh. 25.4 - Prob. 4QQCh. 25.4 - Prob. 5QQCh. 25.4 - Prob. 6QQCh. 25.5 - Prob. 1QQCh. 25.5 - Prob. 2QQCh. 25.5 - Prob. 3QQCh. 25.6 - Prob. 1QQCh. 25.6 - Prob. 2QQCh. 25.6 - Prob. 3QQCh. 25.6 - Prob. 4QQCh. 25.6 - Prob. 5QQCh. 25.6 - Prob. 6QQCh. 25.7 - Prob. 1QQCh. 25.7 - Prob. 2QQCh. 25.7 - Prob. 3QQCh. 25.7 - Prob. 4QQCh. 25.7 - The reducing agent needed in the process of...Ch. 25.7 - Prob. 6QQCh. 25.8 - Prob. 1QQCh. 25.8 - Prob. 2QQCh. 25.9 - Prob. 1QQCh. 25.9 - Prob. 2QQCh. 25.9 - Prob. 3QQCh. 25.9 - Prob. 4QQCh. 25.10 - Which of the following substances cannot be...Ch. 25.10 - Prob. 2QQCh. 25.10 - Which of the following processes occurs within the...Ch. 25.11 - Prob. 1QQCh. 25.11 - Prob. 2QQCh. 25.11 - Prob. 3QQCh. 25 - Indicate whether each of the following aspects of...Ch. 25 - Indicate whether each of the following aspects of...Ch. 25 - Indicate whether each of the following pairings of...Ch. 25 - Prob. 25.4EPCh. 25 - Indicate whether each of the following statements...Ch. 25 - Prob. 25.6EPCh. 25 - Prob. 25.7EPCh. 25 - What is a chylomicron?Ch. 25 - What are the products of the complete hydrolysis...Ch. 25 - What are the major products of the incomplete...Ch. 25 - Prob. 25.11EPCh. 25 - At what location are free fatty acids and...Ch. 25 - Prob. 25.13EPCh. 25 - Prob. 25.14EPCh. 25 - Prob. 25.15EPCh. 25 - Prob. 25.16EPCh. 25 - Prob. 25.17EPCh. 25 - Prob. 25.18EPCh. 25 - Prob. 25.19EPCh. 25 - Prob. 25.20EPCh. 25 - Prob. 25.21EPCh. 25 - Prob. 25.22EPCh. 25 - Prob. 25.23EPCh. 25 - Prob. 25.24EPCh. 25 - Prob. 25.25EPCh. 25 - Prob. 25.26EPCh. 25 - Prob. 25.27EPCh. 25 - Identify the oxidizing agent needed in Step 3 of a...Ch. 25 - Prob. 25.29EPCh. 25 - Prob. 25.30EPCh. 25 - Prob. 25.31EPCh. 25 - Prob. 25.32EPCh. 25 - Prob. 25.33EPCh. 25 - Prob. 25.34EPCh. 25 - Prob. 25.35EPCh. 25 - Prob. 25.36EPCh. 25 - Prob. 25.37EPCh. 25 - Prob. 25.38EPCh. 25 - Prob. 25.39EPCh. 25 - Prob. 25.40EPCh. 25 - Prob. 25.41EPCh. 25 - Prob. 25.42EPCh. 25 - How many turns of the -oxidation pathway would be...Ch. 25 - How many turns of the -oxidation pathway would be...Ch. 25 - Prob. 25.45EPCh. 25 - Prob. 25.46EPCh. 25 - Prob. 25.47EPCh. 25 - Prob. 25.48EPCh. 25 - Prob. 25.49EPCh. 25 - Explain why fatty acids cannot serve as fuel for...Ch. 25 - Prob. 25.51EPCh. 25 - Prob. 25.52EPCh. 25 - Prob. 25.53EPCh. 25 - Prob. 25.54EPCh. 25 - Prob. 25.55EPCh. 25 - Prob. 25.56EPCh. 25 - Prob. 25.57EPCh. 25 - Prob. 25.58EPCh. 25 - Prob. 25.59EPCh. 25 - Prob. 25.60EPCh. 25 - Prob. 25.61EPCh. 25 - Why does a deficiency of carbohydrates in the diet...Ch. 25 - Prob. 25.63EPCh. 25 - Prob. 25.64EPCh. 25 - Prob. 25.65EPCh. 25 - Prob. 25.66EPCh. 25 - Prob. 25.67EPCh. 25 - Prob. 25.68EPCh. 25 - Prob. 25.69EPCh. 25 - Prob. 25.70EPCh. 25 - Prob. 25.71EPCh. 25 - Prob. 25.72EPCh. 25 - Prob. 25.73EPCh. 25 - Prob. 25.74EPCh. 25 - Prob. 25.75EPCh. 25 - Severe ketosis situations produce acidosis....Ch. 25 - Prob. 25.77EPCh. 25 - Prob. 25.78EPCh. 25 - Prob. 25.79EPCh. 25 - Prob. 25.80EPCh. 25 - Prob. 25.81EPCh. 25 - Prob. 25.82EPCh. 25 - Prob. 25.83EPCh. 25 - Prob. 25.84EPCh. 25 - Prob. 25.85EPCh. 25 - Prob. 25.86EPCh. 25 - Prob. 25.87EPCh. 25 - Prob. 25.88EPCh. 25 - Prob. 25.89EPCh. 25 - Prob. 25.90EPCh. 25 - Prob. 25.91EPCh. 25 - Prob. 25.92EPCh. 25 - Prob. 25.93EPCh. 25 - Prob. 25.94EPCh. 25 - What role does molecular oxygen, O2, play in fatty...Ch. 25 - Prob. 25.96EPCh. 25 - Prob. 25.97EPCh. 25 - Prob. 25.98EPCh. 25 - Prob. 25.99EPCh. 25 - Prob. 25.100EPCh. 25 - Prob. 25.101EPCh. 25 - Prob. 25.102EPCh. 25 - Prob. 25.103EPCh. 25 - Prob. 25.104EPCh. 25 - Prob. 25.105EPCh. 25 - Prob. 25.106EPCh. 25 - Prob. 25.107EPCh. 25 - Prob. 25.108EPCh. 25 - Prob. 25.109EPCh. 25 - Prob. 25.110EPCh. 25 - Prob. 25.111EPCh. 25 - Prob. 25.112EPCh. 25 - Prob. 25.113EPCh. 25 - Prob. 25.114EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
- 1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning