
Concept explainers
(a)
Interpretation:
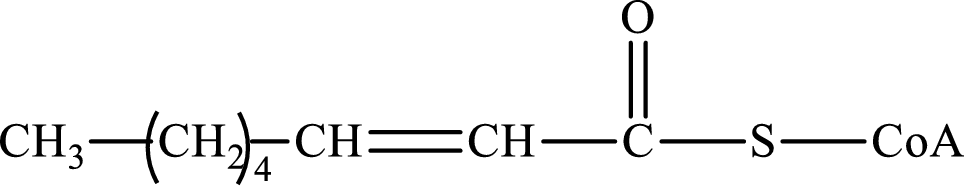
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
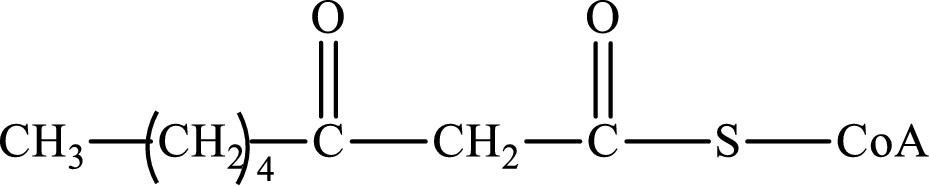
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
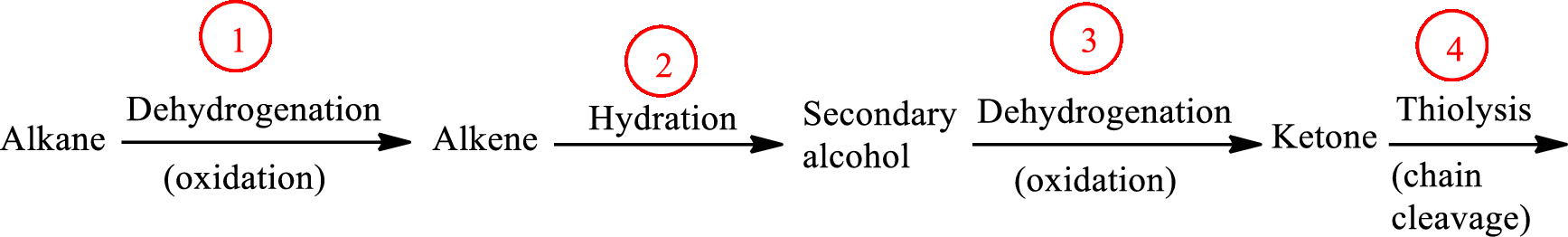
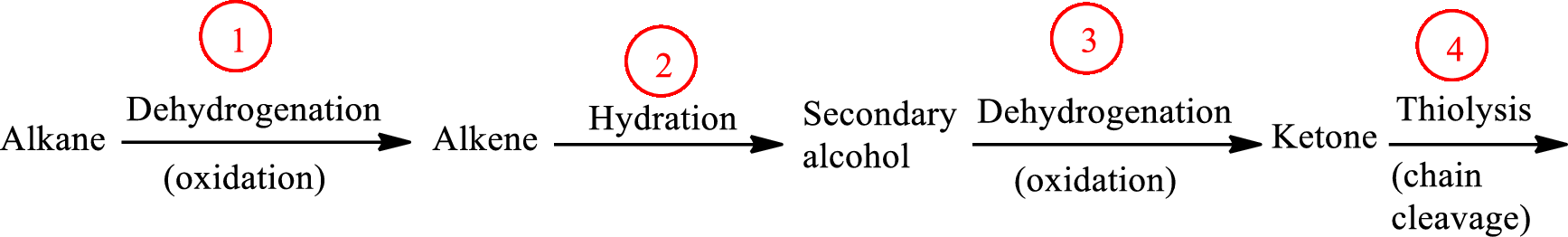
The functional group change in the β-oxidation pathway is as follows:
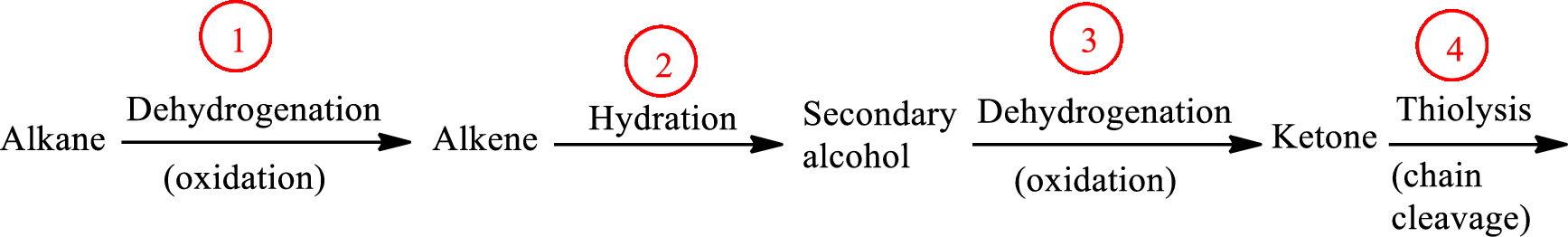
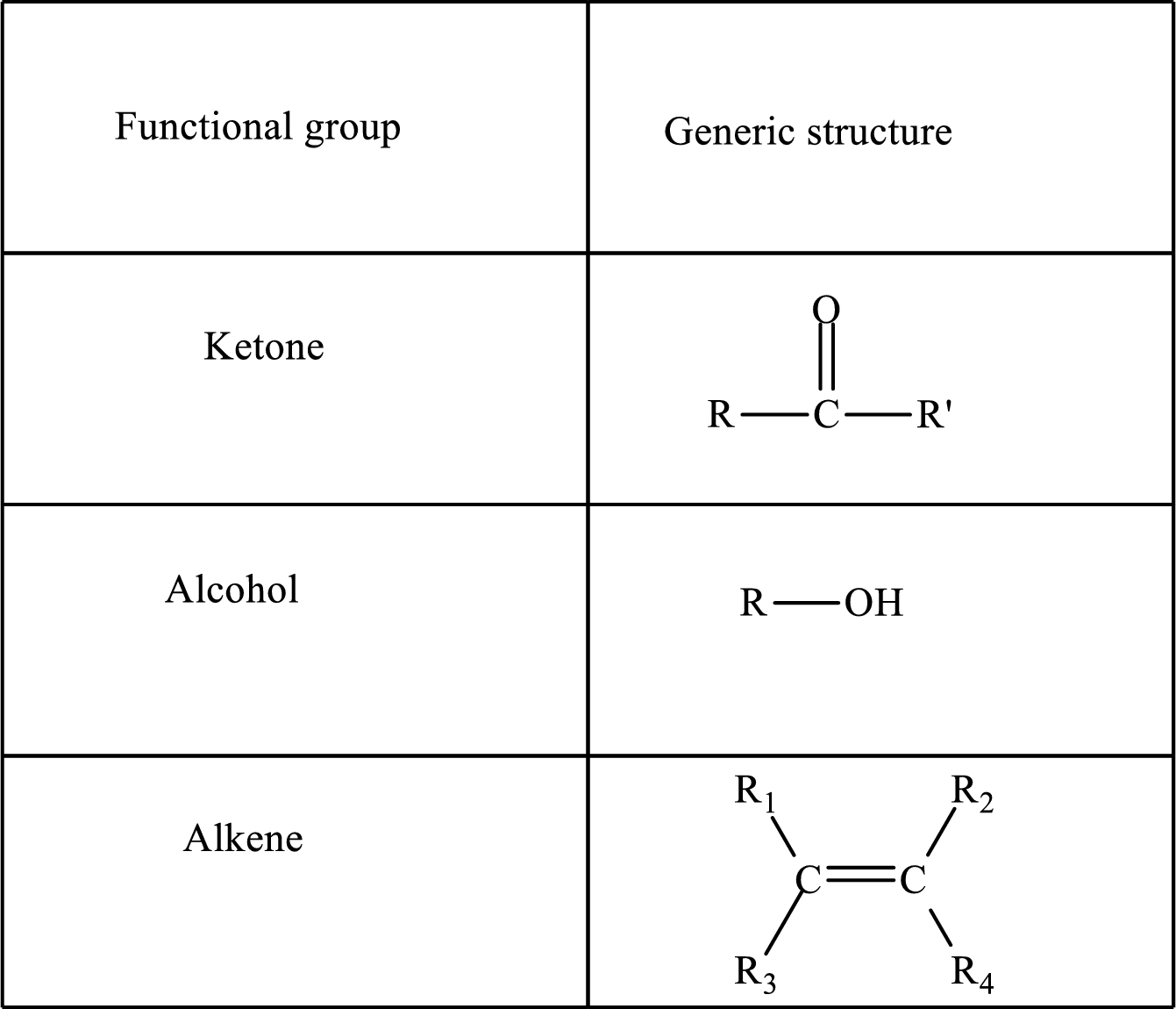
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
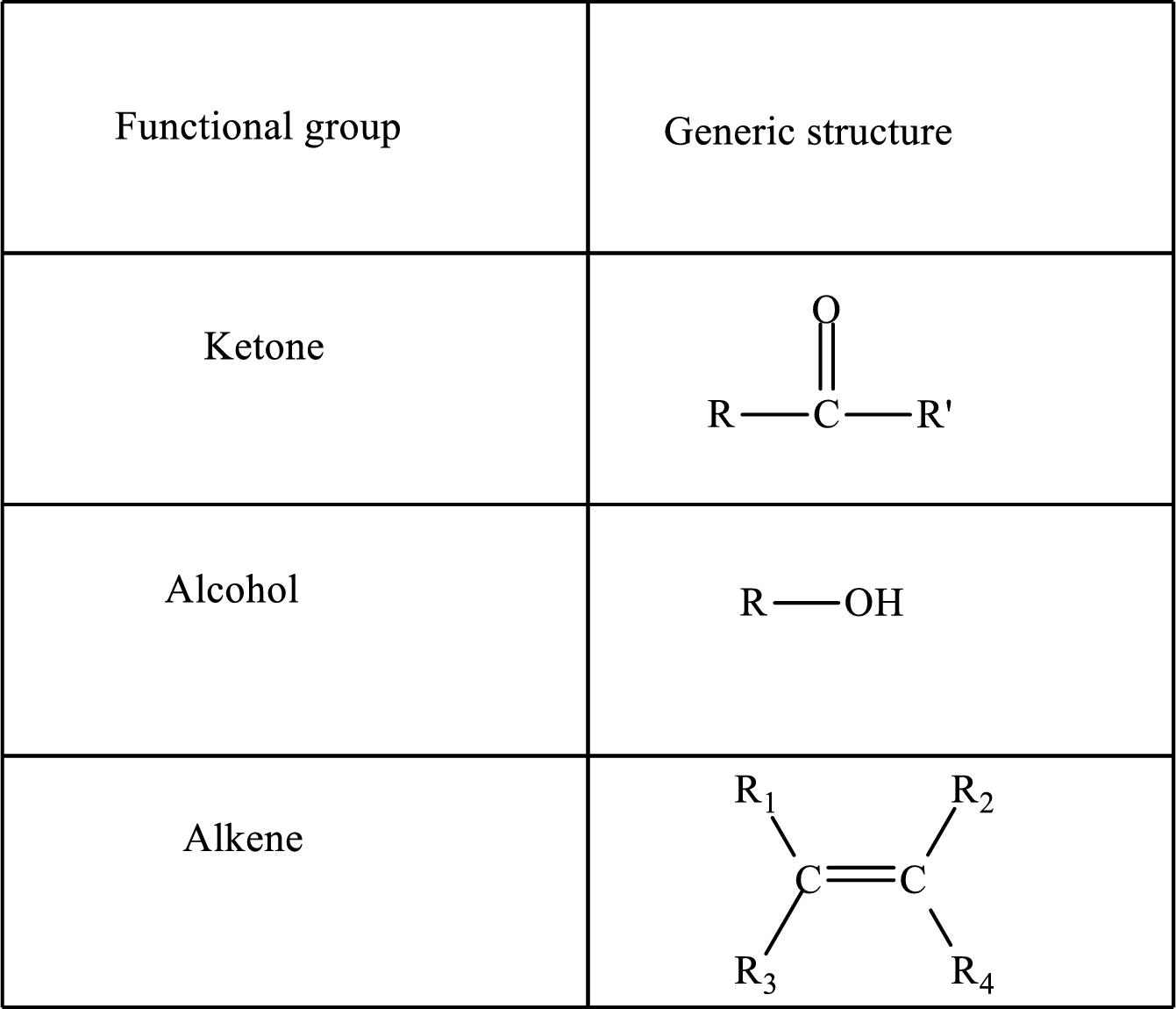
Here, R and R’ represent an alkyl group. In
(b)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
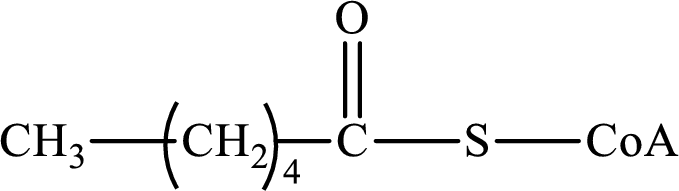
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
The functional group change in the β-oxidation pathway is as follows:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
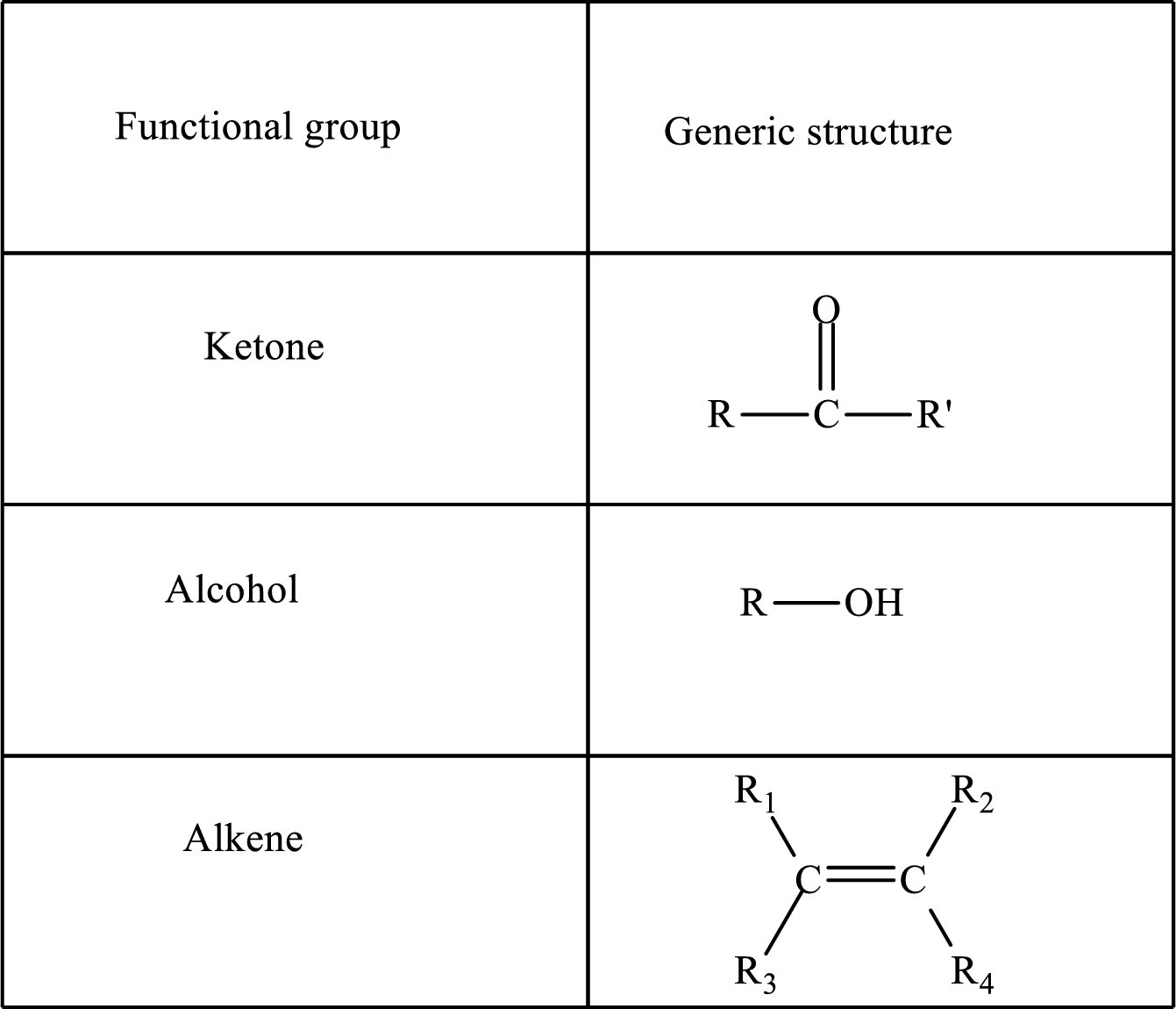
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
(c)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
The functional group change in the β-oxidation pathway is as follows:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
(d)
Interpretation:
The step (of steps 1 through 4) and turn (second or third) of the β-oxidation pathway in which the following compound is encountered as a reactant if the degraded fatty acid is decanoic acid has to be determined.
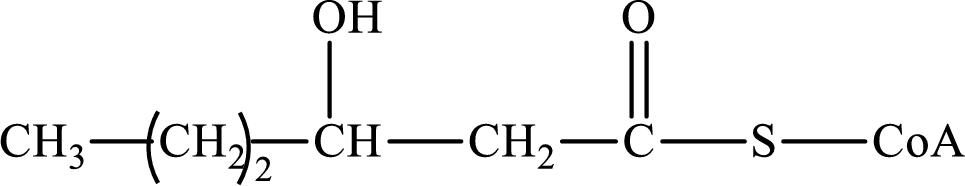
Concept introduction:
The β-oxidation pathway is defined as a repetitive series of four biochemical reactions in which acyl CoA is degraded to acetyl CoA by the removal of two carbon atoms at a time. NADH and FADH2 are also produced in this pathway.
The functional group change in the β-oxidation pathway is as follows:
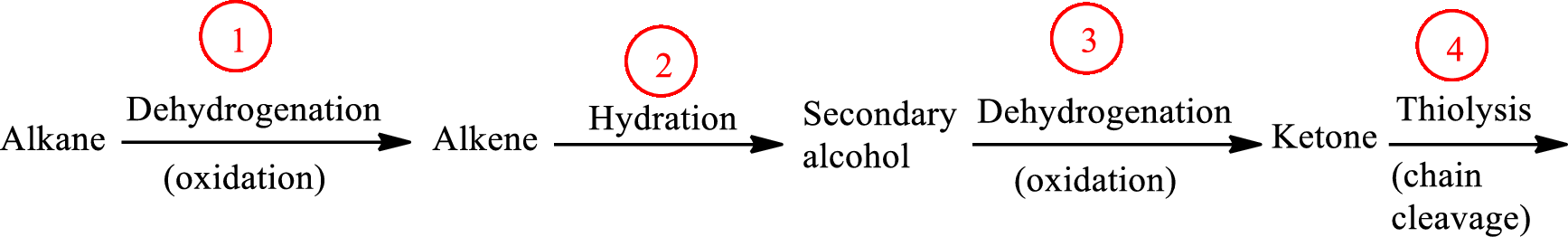
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
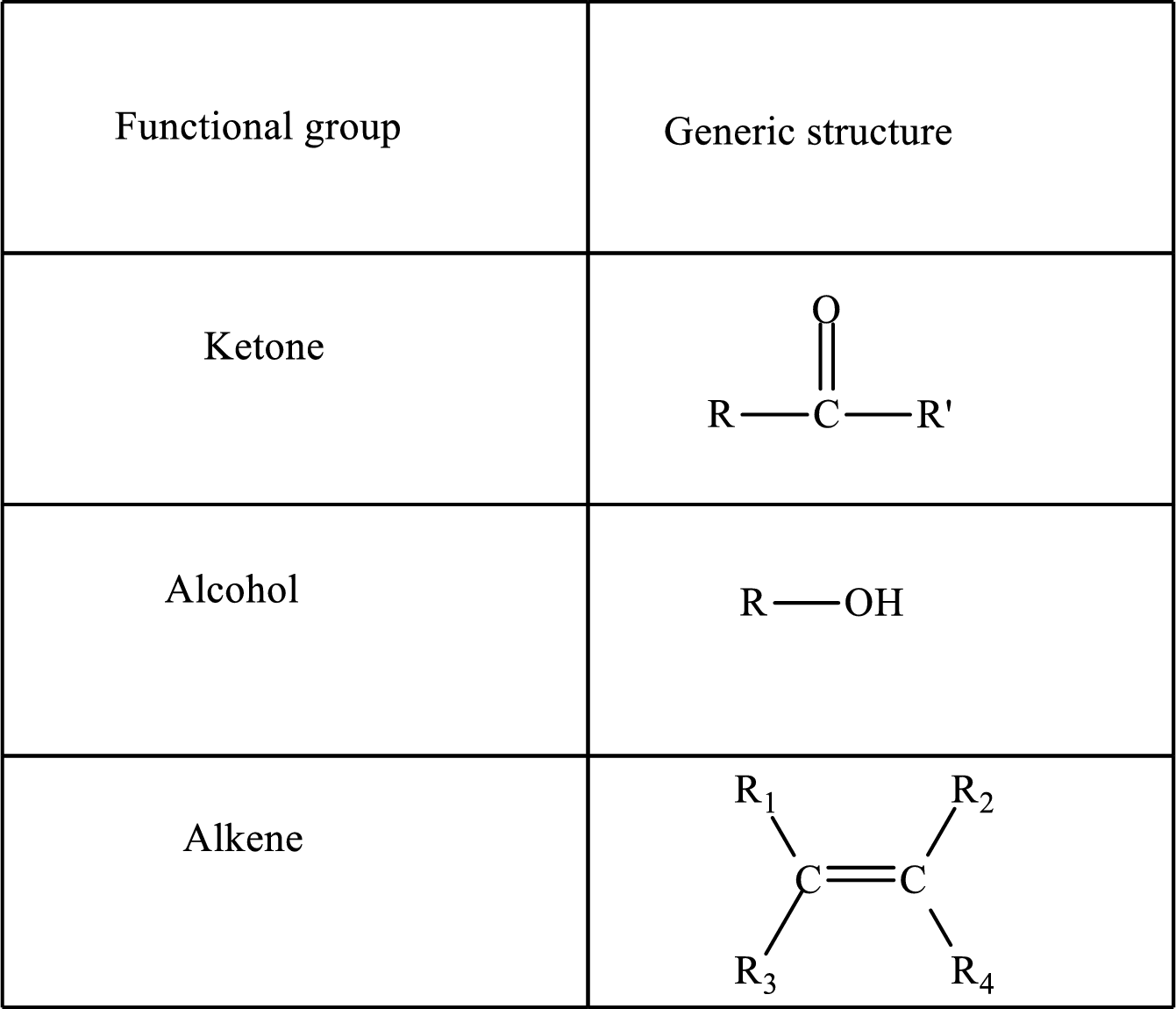
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- The quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardIf the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forward
- If the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





