
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24.6, Problem 24.7P
Interpretation Introduction
Interpretation:
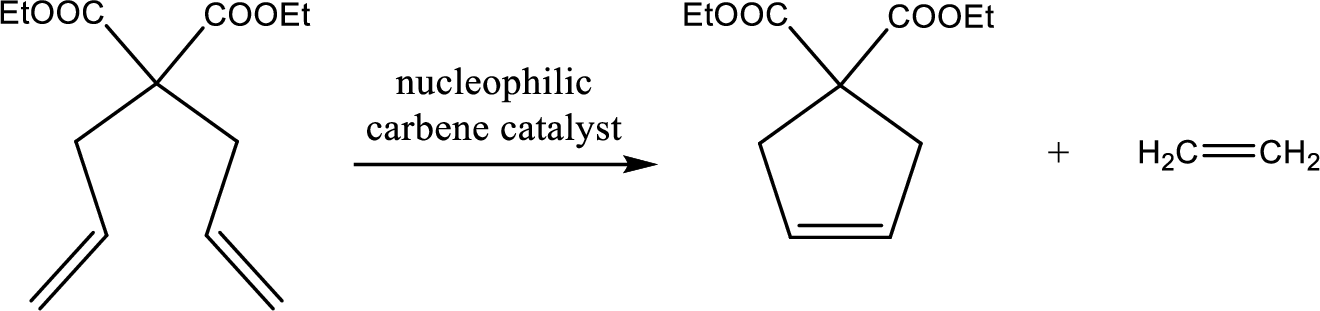
The product of the given reaction has to be shown.
Concept Introduction:
Ring-closing alkene metathesis involves two terminal
The catalyst with
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.
Pls help.
13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions
would be:
a. 1:1
b. 2:3
c. 3:2
d. 2:1
14) The pH of a 0.05 M solution of HCl(aq) at 25°C is
15) The pH of a 0.20 M solution of KOH at 25°C is
Chapter 24 Solutions
Organic Chemistry
Ch. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.4 - Prob. 24.3PCh. 24.5 - Show how the following compound can be prepared...Ch. 24.5 - Prob. 24.5PCh. 24.5 - Prob. 24.6PCh. 24.6 - Prob. 24.7PCh. 24 - Prob. 24.8PCh. 24 - Prob. 24.9PCh. 24 - Prob. 24.10P
Ch. 24 - Treatment of cyclohexene with iodobenzene under...Ch. 24 - Prob. 24.12PCh. 24 - Prob. 24.13PCh. 24 - The aryl diene undergoes sequential Heck reactions...Ch. 24 - Heck reactions take place with alkynes as well as...Ch. 24 - Prob. 24.16PCh. 24 - The following transformation involves a series of...Ch. 24 - Show the sequence of Heck reactions by which the...Ch. 24 - Prob. 24.19PCh. 24 - Write the steps that are critical in the following...Ch. 24 - Prob. 24.21PCh. 24 - Prob. 24.22PCh. 24 - Prob. 24.23PCh. 24 - Show how the following compound could be prepared...Ch. 24 - It is typically very difficult to do a...Ch. 24 - The compound eutypine is an antibacterial agent...Ch. 24 - Prob. 24.27PCh. 24 - Prob. 24.28PCh. 24 - Prob. 24.29PCh. 24 - Prob. 24.30PCh. 24 - Prob. 24.31PCh. 24 - Prob. 24.32PCh. 24 - Prob. 24.33PCh. 24 - The following transformation can be accomplished...Ch. 24 - Prob. 24.35PCh. 24 - Prob. 24.36PCh. 24 - Prob. 24.37PCh. 24 - Prob. 24.38PCh. 24 - E. J. Coreys 1964 total synthesis of...Ch. 24 - Prob. 24.40P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help.arrow_forwardPls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward
- 7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward11) The Ksp expression for copper (II) sulfate is: a. [Cu2+][SO4²¯] b. [Cu²+]² [SO4²]² c. [Cu²+]²[SO4²] d. [CuSO4] 12) Which of the following is true about a chemical system in equilibrium? a. All chemical reactions have stopped b. The concentration of reactants is equal to the concertation of products c. The forward and reverse reaction rates become equal d. The system will remain at equilibrium regardless of any external factorsarrow_forward21) Explain the difference between the rate of a reaction and the extent of a reaction. Why are both of these concepts important, if you are a chemical engineer that is trying to develop a process to produce a large volume of a specific type of chemical compound?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY