
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 24.11P
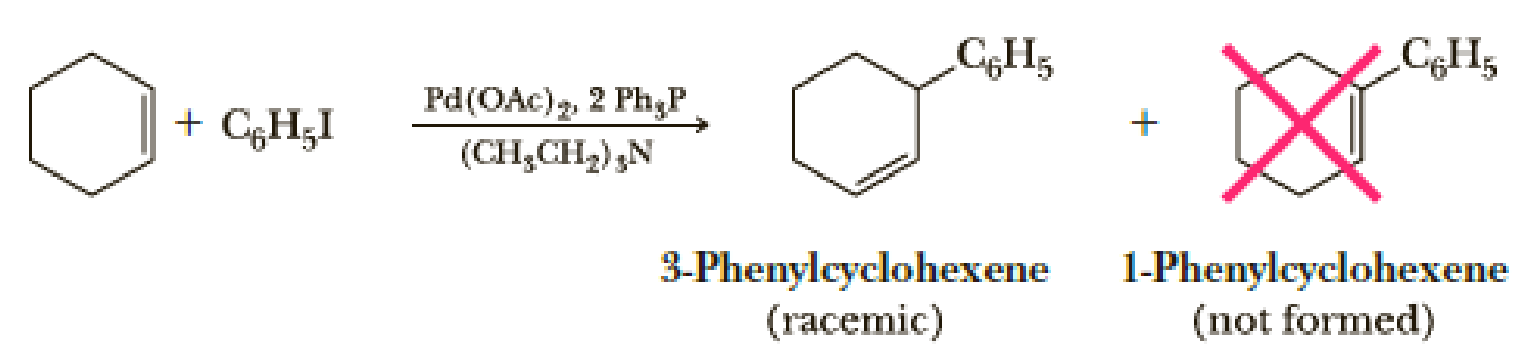
Treatment of cyclohexene with iodobenzene under the conditions of the Heck reaction might be expected to give 1-phenylcyclohexene. The exclusive product, however, is 3-phenylcyclohexene. Account for the formation of this product.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Consider the Grignard reaction below to answer the following questions.
A
Mgar
1. ether
+
MyC
CH3
2H3O
C
B
a. The electrophile in this reaction is:
b. The nucleophile in this reaction is:
c. The alcohol product can be classified as a:
a.
1° alcohol
b. 2° alcohol
C.
3° alcohol
d. 4° alcohol
HO
CH3
CH
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show
all relevant stereochemistry
A.
CH₂OH
PCC
CH2Cl2
HOO
B.
H
KCN
HCN
of b
C.
1. CH,MgBr, ether
2 HO*
D. Choose the BEST reagent for carrying out each of the following conversions.
CO₂CH3
CO₂CH3
OH
CO₂H
сон
ن نے
a.
LiAlH4, ether at abinayo iss c
b. NaBH4, ethanol
C.
CrO3, pyridine
d. H₂/Pd d
notsiol
Choose the best reagent for carrying out the following reactions from the list below. Place the letter of
the reagent(s) in the box over the reaction arrow. Use only one letter per box.
OH
OH
CH
CH
CH3
CHS
CH3
f
OH
OCH 3
H
A.
NaH, then CHI
B.
C.
m-ClC6H4COзH
D.
E.
warm H2SO4/H₂O
F.
G.
H₂/Pd
H.
I.
Cl₂, H₂O
J.
NaOCH3, CH3OH
CH3MgBr in ether, then H3O+
Hg(O2CCF3)2, CH3OH
PCC, CH2Cl2
LiAlH4 in ether, then H3O+
Chapter 24 Solutions
Organic Chemistry
Ch. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.4 - Prob. 24.3PCh. 24.5 - Show how the following compound can be prepared...Ch. 24.5 - Prob. 24.5PCh. 24.5 - Prob. 24.6PCh. 24.6 - Prob. 24.7PCh. 24 - Prob. 24.8PCh. 24 - Prob. 24.9PCh. 24 - Prob. 24.10P
Ch. 24 - Treatment of cyclohexene with iodobenzene under...Ch. 24 - Prob. 24.12PCh. 24 - Prob. 24.13PCh. 24 - The aryl diene undergoes sequential Heck reactions...Ch. 24 - Heck reactions take place with alkynes as well as...Ch. 24 - Prob. 24.16PCh. 24 - The following transformation involves a series of...Ch. 24 - Show the sequence of Heck reactions by which the...Ch. 24 - Prob. 24.19PCh. 24 - Write the steps that are critical in the following...Ch. 24 - Prob. 24.21PCh. 24 - Prob. 24.22PCh. 24 - Prob. 24.23PCh. 24 - Show how the following compound could be prepared...Ch. 24 - It is typically very difficult to do a...Ch. 24 - The compound eutypine is an antibacterial agent...Ch. 24 - Prob. 24.27PCh. 24 - Prob. 24.28PCh. 24 - Prob. 24.29PCh. 24 - Prob. 24.30PCh. 24 - Prob. 24.31PCh. 24 - Prob. 24.32PCh. 24 - Prob. 24.33PCh. 24 - The following transformation can be accomplished...Ch. 24 - Prob. 24.35PCh. 24 - Prob. 24.36PCh. 24 - Prob. 24.37PCh. 24 - Prob. 24.38PCh. 24 - E. J. Coreys 1964 total synthesis of...Ch. 24 - Prob. 24.40P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forwardIn the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forwardQUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forward
- If the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forwardoalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forward
- Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forward
- By malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forwardDraw the formula for 3-chlorobenzoic acetic anhydride.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY