
Concept explainers
(a)
Interpretation:
The function of the sodium hydride has to be determined in the 1st step along with the
Concept introduction:
Aromaticity:
Aromaticity is a property of cyclic, planar structures with a ring of resonance bonds that gives increased stability compared to the other geometric or connective arrangements with the same set of atoms. Aromatic molecules are very stable and do not easily break up to take part in reactions.
There are some rules for a compound to be aromatic i.e. they have to be planar, cyclic and must contain
(a)
Explanation of Solution
In the given question the 1st step is,
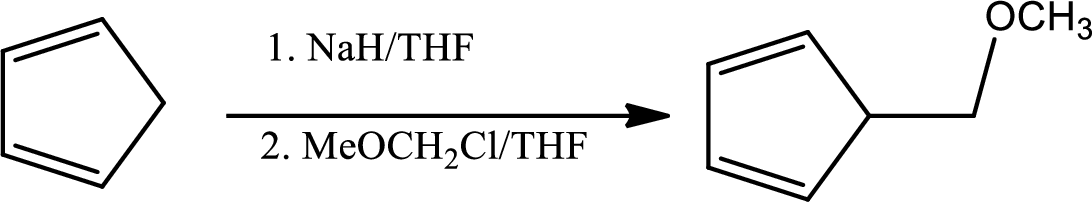
The mechanism as follows,
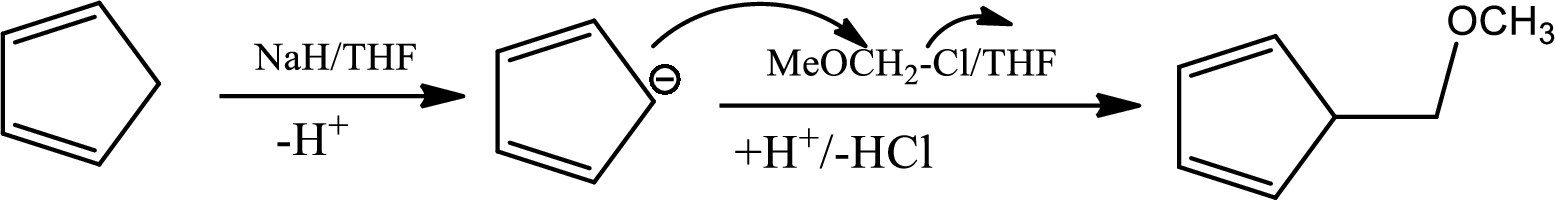
Here
The
(b)
Interpretation:
The reaction pathway by which B is converted to C that has to be identified.
Concept introduction:
Diels Alder reaction:
The Diels-Alder reaction is a
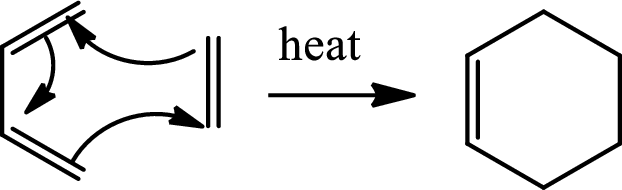
(b)
Explanation of Solution
According to the question,
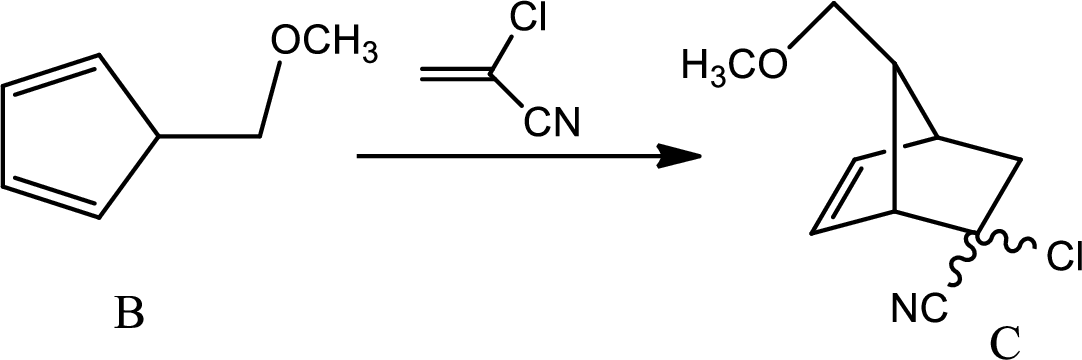
Here B is the diene and the dienophile consists of electron withdrawing groups.
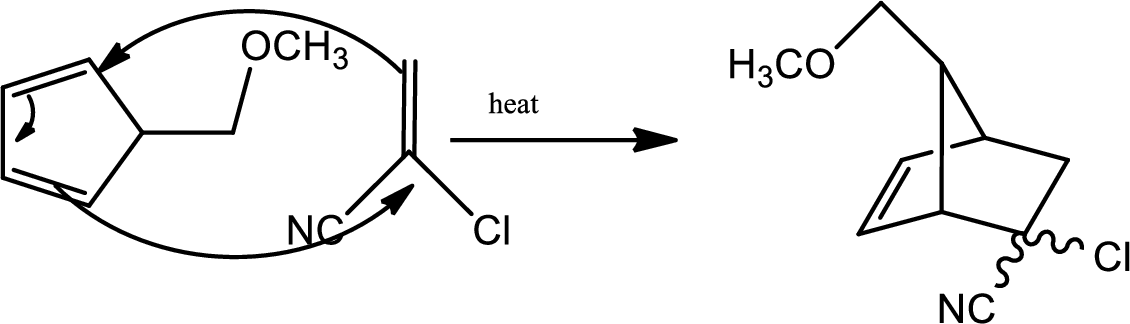
Thus via Diels Alder reaction the product is obtained.
(c)
Interpretation:
The role of the carbon dioxide added to the reaction mixture in step 2 of the conversion of (E) to (F) has to be determined.
Concept introduction:
Lactone formation:
Lactones are cyclic carboxylic esters that are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids which takes place spontaneously when th ering that is formed is five or six membered. The reaction pathway is as follows,
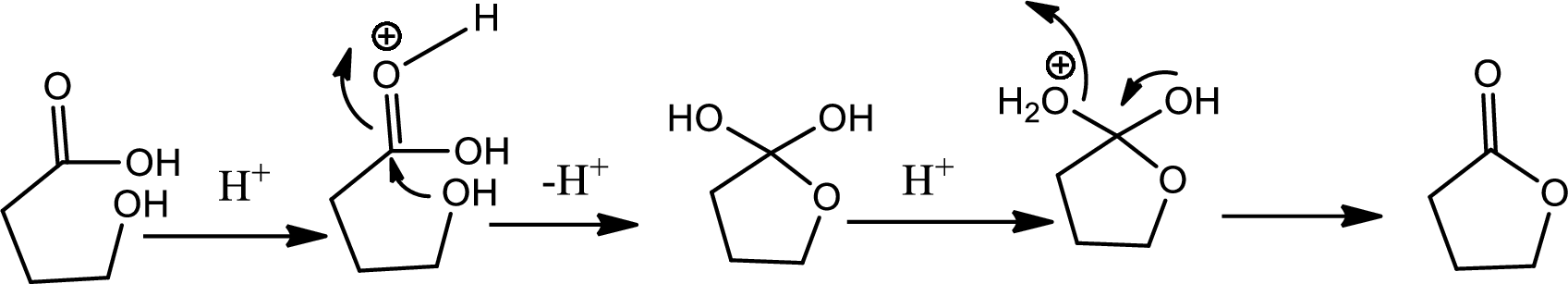
(c)
Explanation of Solution
The reaction given is,
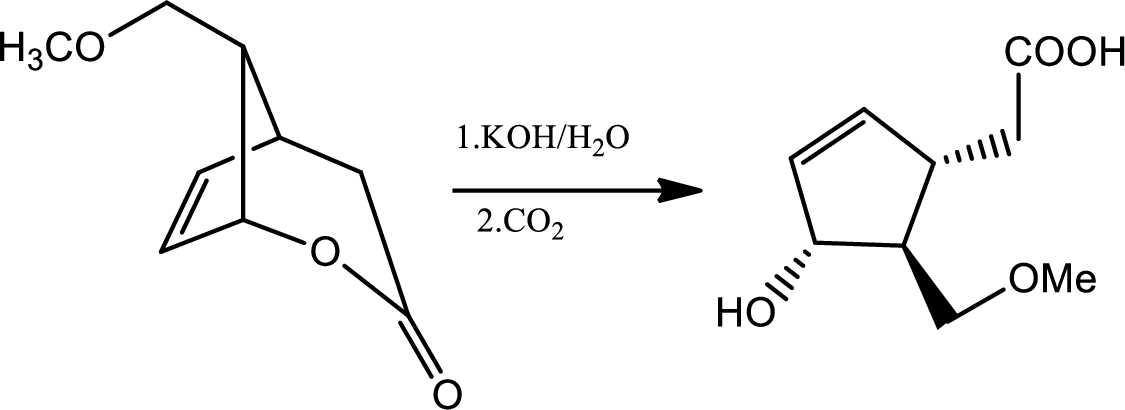
In this reaction in the presence of base lactone is hydrolised to give back acid and alcohol. Now if the amount of base is more in medium then again the backward reaction will be favoured and lactone will be formed again. So the reactant will be formed again. Hence to maintain the
Carbon dioxide reacts with water to give mild amount of carbonic acid that dissociates in water to a little extent to give less amount of proton which is sufficient to balance the
(d)
Interpretation:
A reaction pathway has to suggest for the conversion of (H) to (I) via radical mechanism.
Concept introduction:
Radical reaction:
A free radical reaction is a chemical reaction involving free radicals. Radical reaction contain three steps that are respectively chain initiation, chain propagation and chain termination.
(d)
Explanation of Solution
The reaction given,
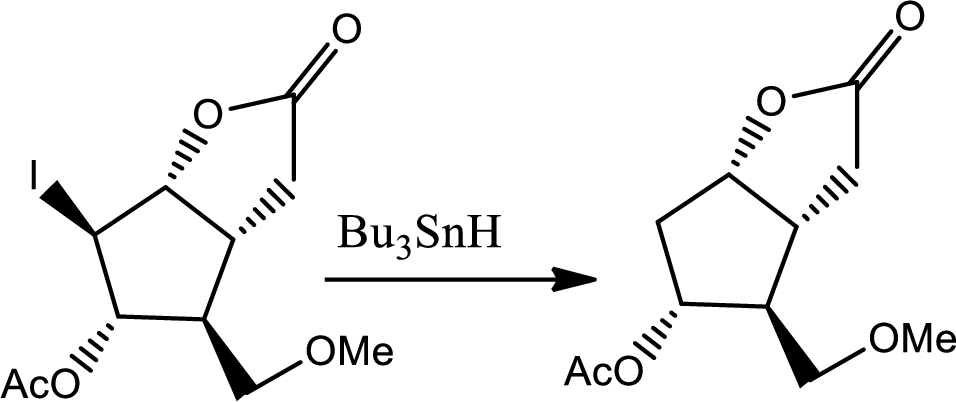
According to the question, the reaction proceeds via radical pathway and the 1st step involves a radical initiator to form
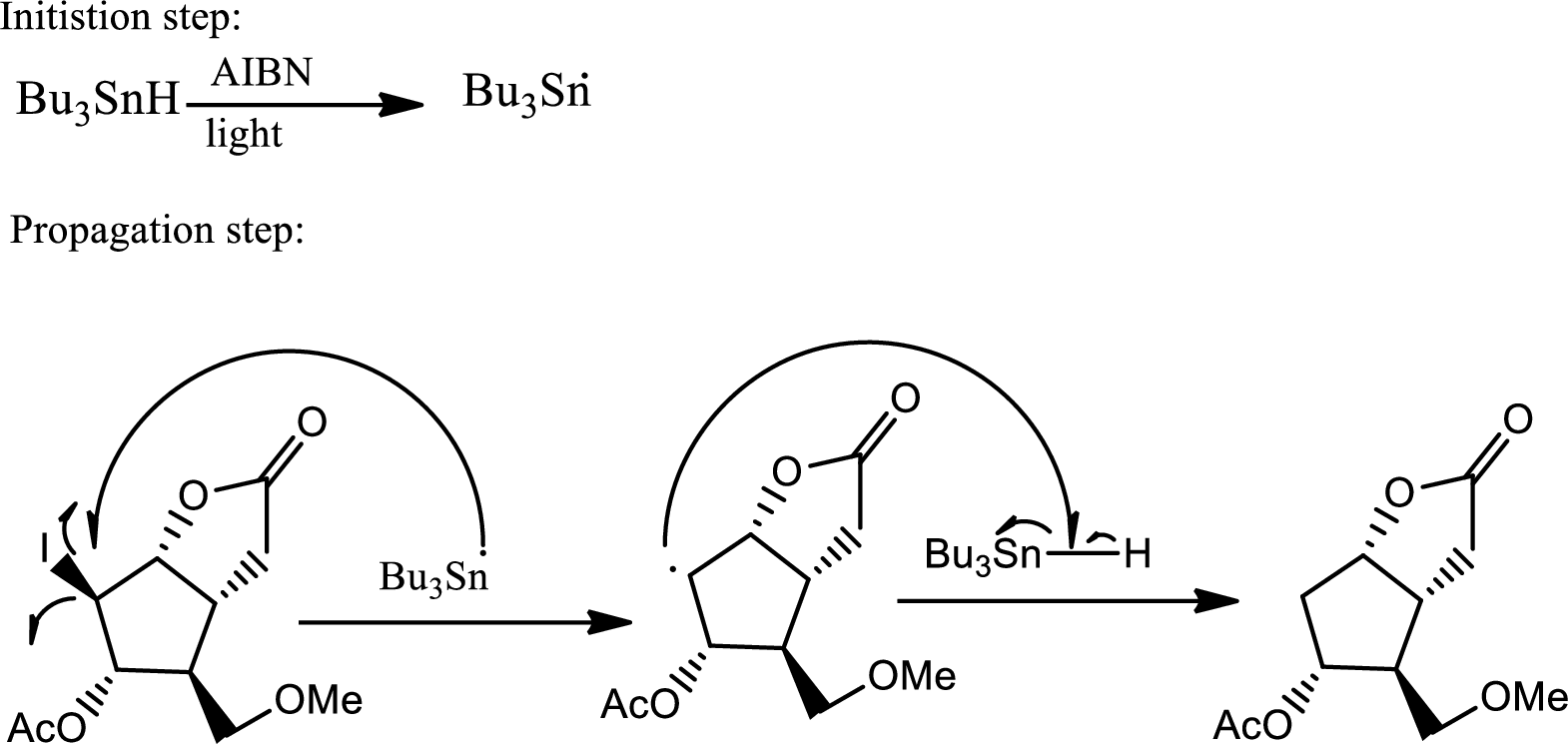
As a radical initiator AIBN is used. The reaction undergoes via two steps which are initiation and propagation respectively. Initiation step involves a radical initiator to form
(e)
Interpretation:
The steps in which the chiral centers of Corey lactone can be determined has to be shown with the mechanism.
Concept introduction:
Chiral centre:
Chiral centre is defined as an atom bonded to four different chemical species. It is a stereo centre that holds the atom in such way that the structure may not be superimposable to its mirror image. They give optical isomerism.
(e)
Explanation of Solution
In the question it is given that the Corey lactone has four chiral centers which are pointed below,
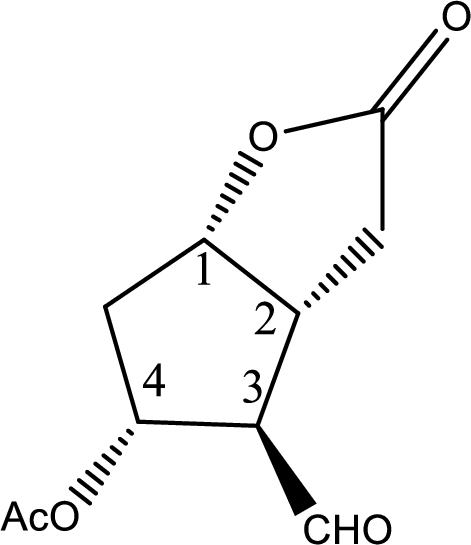
Now the steps where the chiral centers can be distinguished are given below,
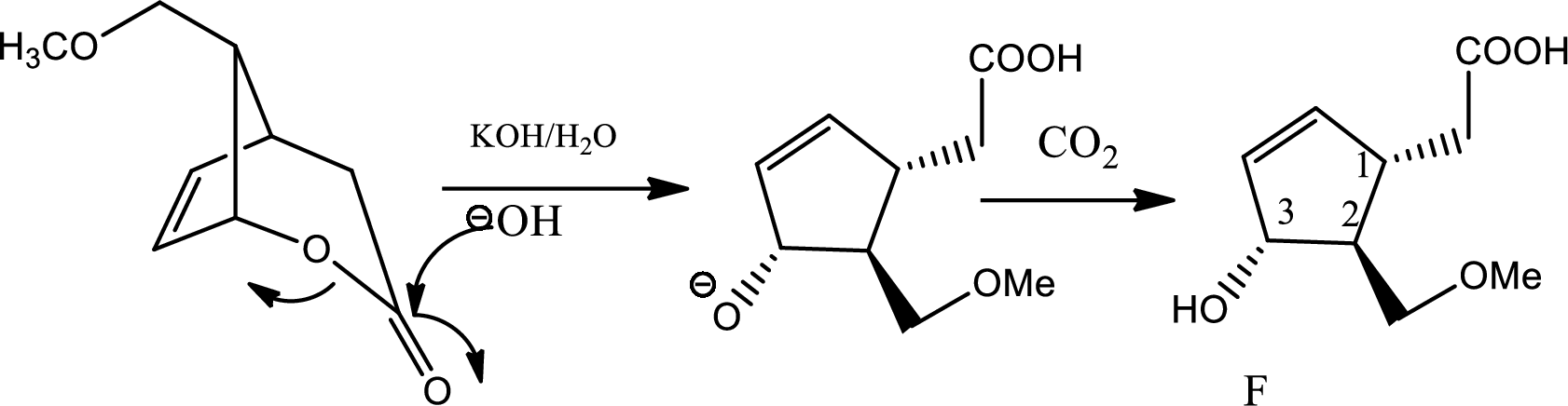
In this step due to hydrolisation of lactone, ring opening occurs along with the formation of acid and alcohol.
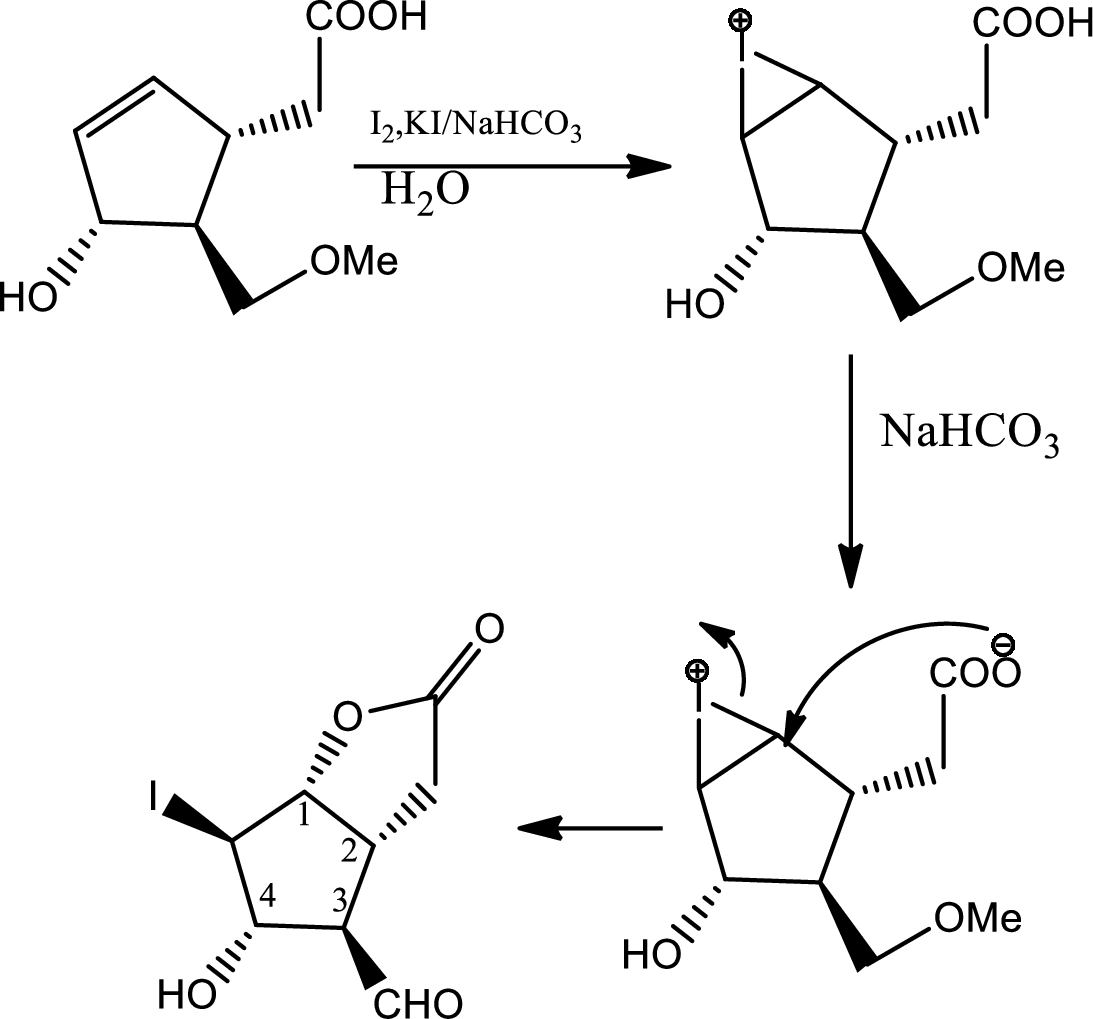
In this step normal
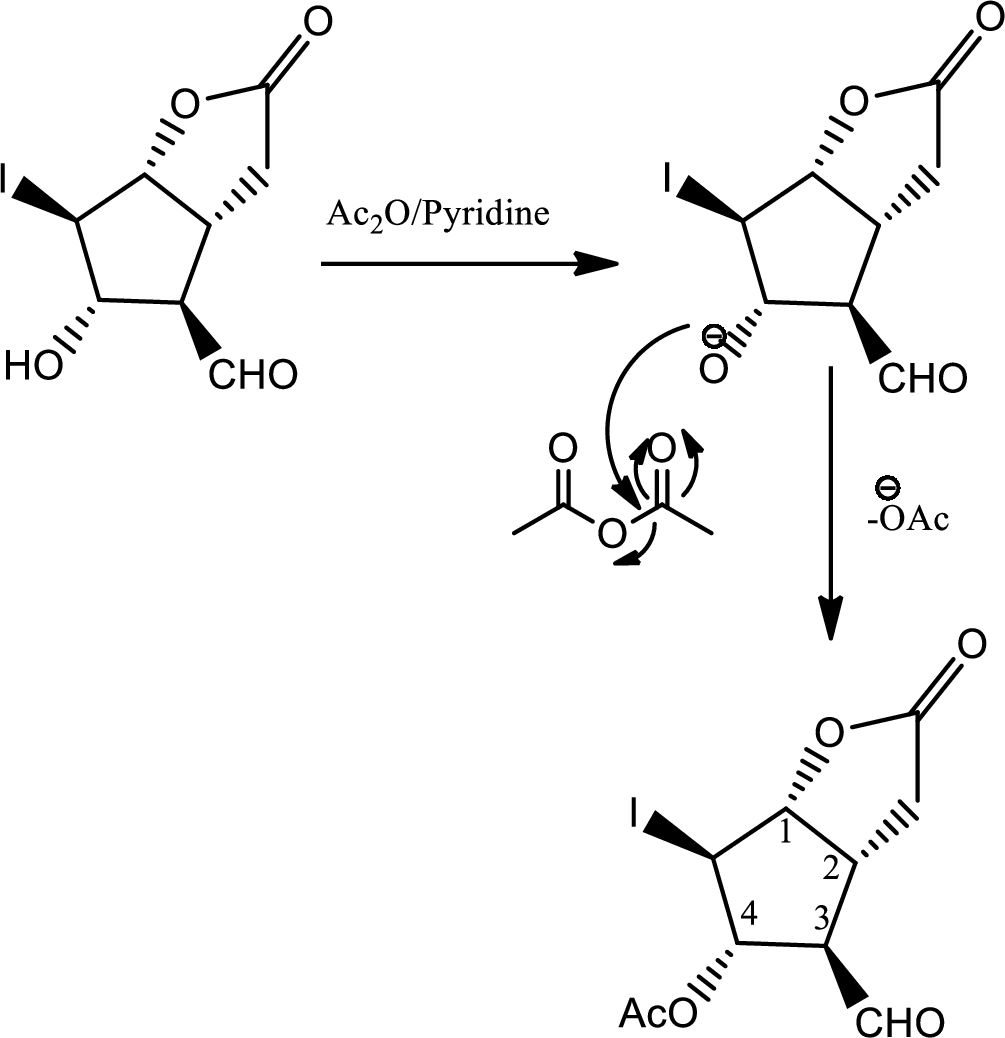
In this step normal acid base reaction occurs followed by normal
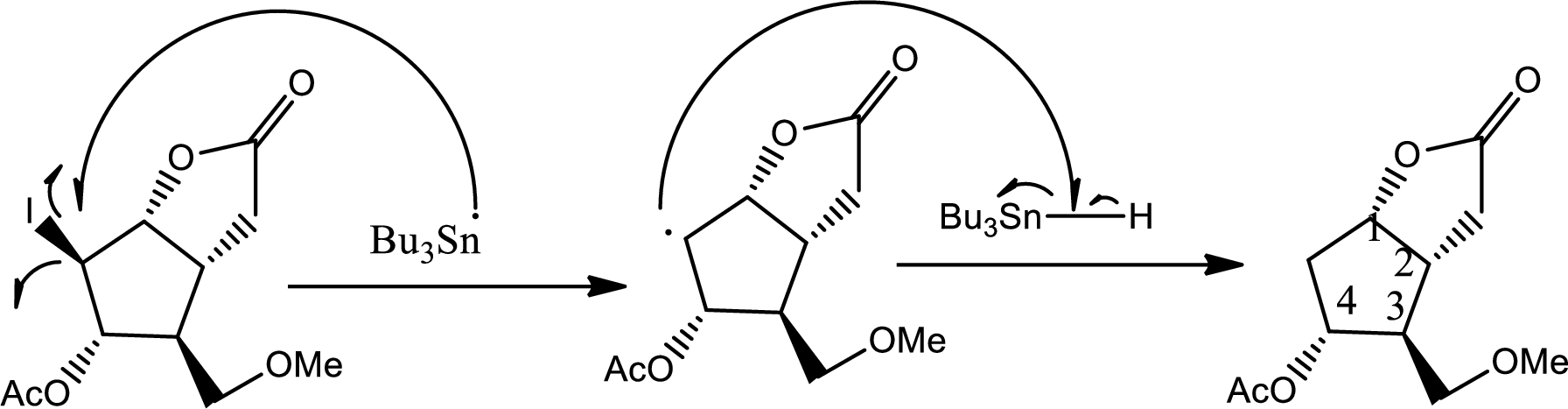
Via radical mechanism this step occurs to break the carbon-halogen bond.
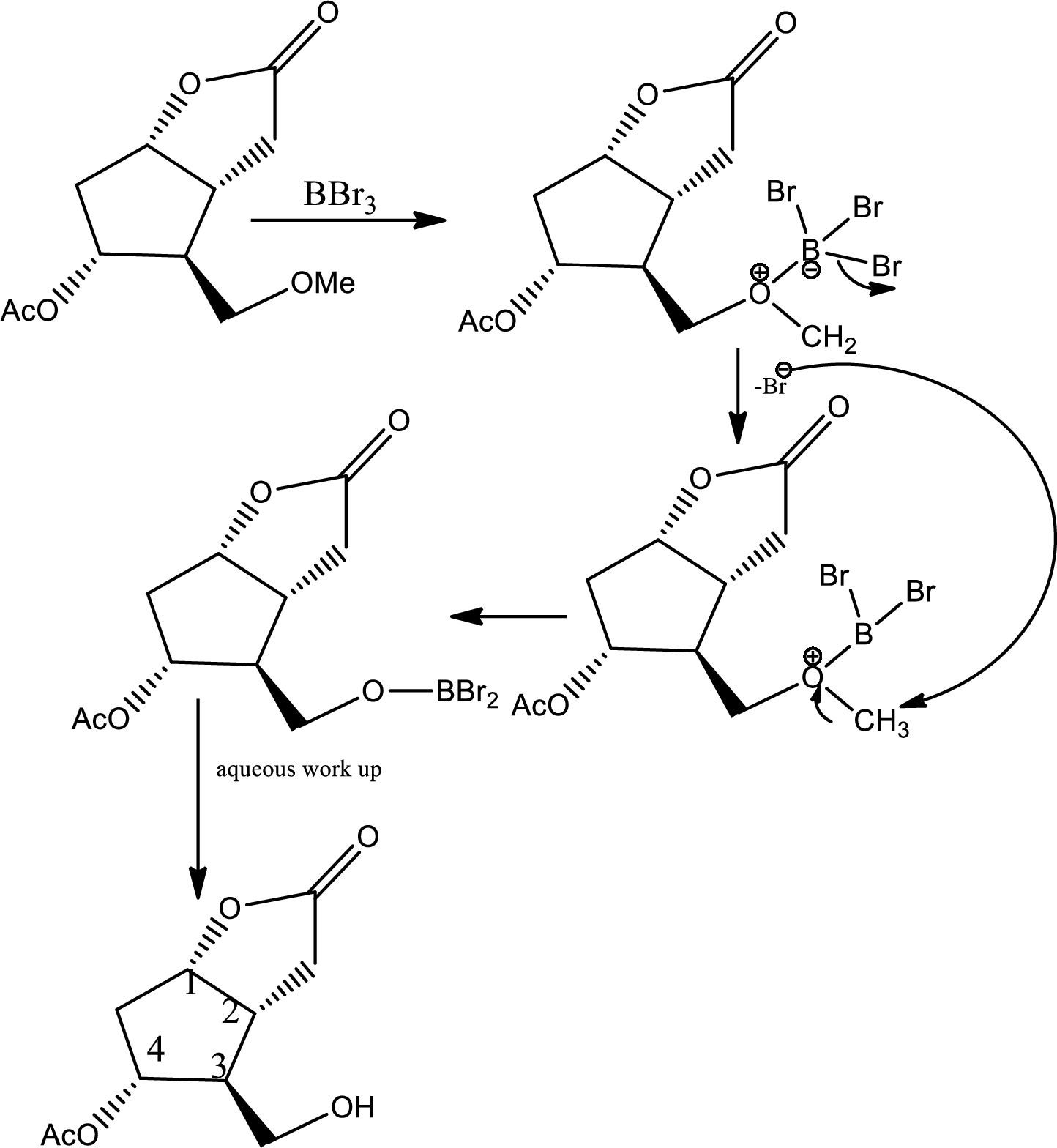
Here the ether is converted to alcohol.
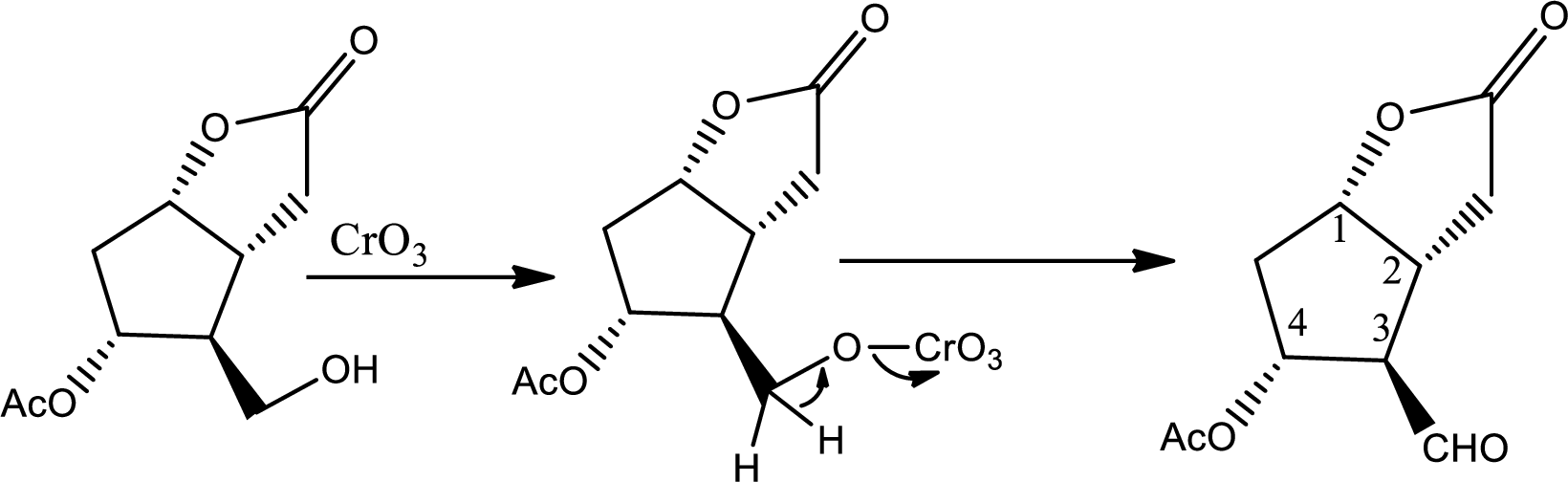
Here alcohol is oxidized to
In all these steps the chiral centres can be distinguished.
(f)
Interpretation:
The definition of resolution has to be given along with the rationale for using a chiral, enantiomerically pure
Concept introduction:
Resolution:
Chiral resolution in stereochemistry is a process for the seperation of racemic compounds into their enantiomers. Now racemic mixture is the mixture that has equal amounts of left and right handed enantiomers of the chiral molecule.
(f)
Explanation of Solution
According to the question the compound F formed is resolved by (+)-ephedrine. The structures of compound (F) and (-)-ephedrine are given below,
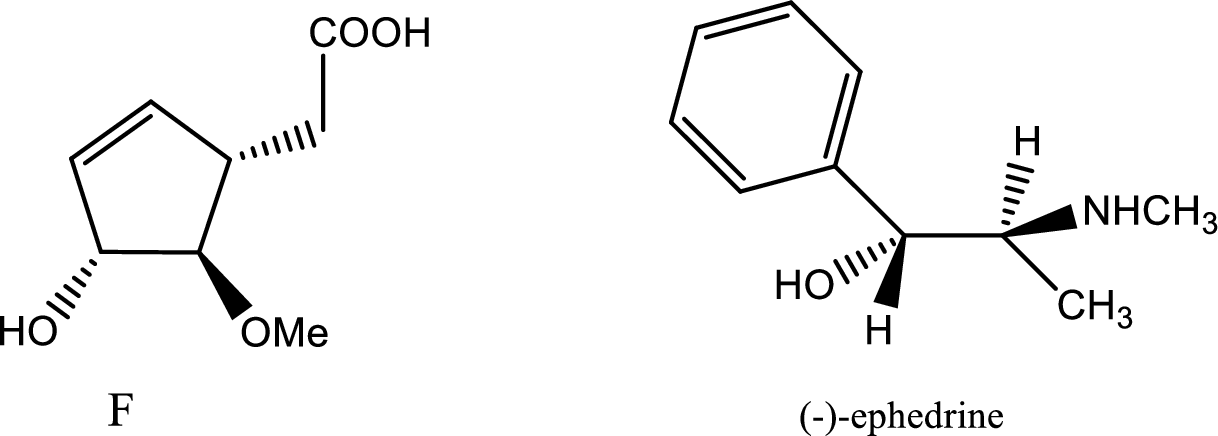
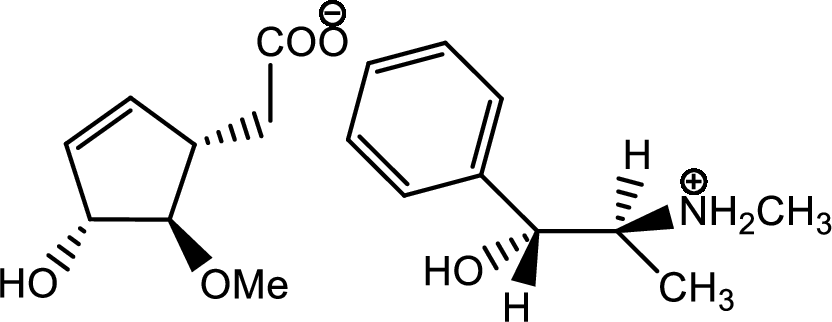
The process of racemisation follows acid base reaction between compound F and (+)-ephedrine. The two enantiomers can be converted to two diastereomeric salts those are [(+)(+)] and [(-)(+)]. Now being diastereomers they can be easily separated as diastereomers have different physical properties. Thus racemisation process works to separate two enantiomers.
One of the salts is shown below,
(g)
Interpretation:
The mechanism for the reaction pathway from D to E that undergoes via Baeyer-Villiger oxidation has to be shown.
Concept introduction:
Baeyer-Villiger oxidation:
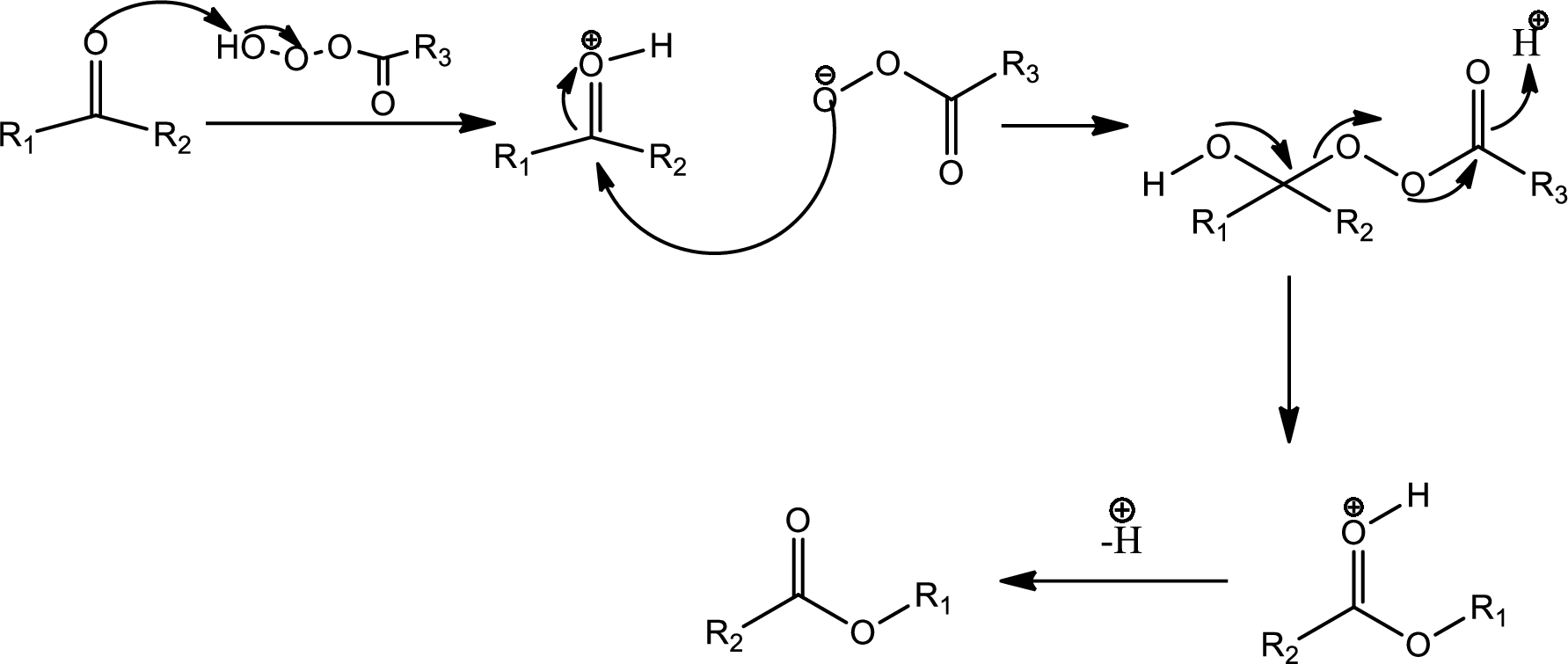
The Baeyer-Villiger oxidation is an organic rearrangement reaction that forms ester from a
(g)
Explanation of Solution
The reaction given in the question is,
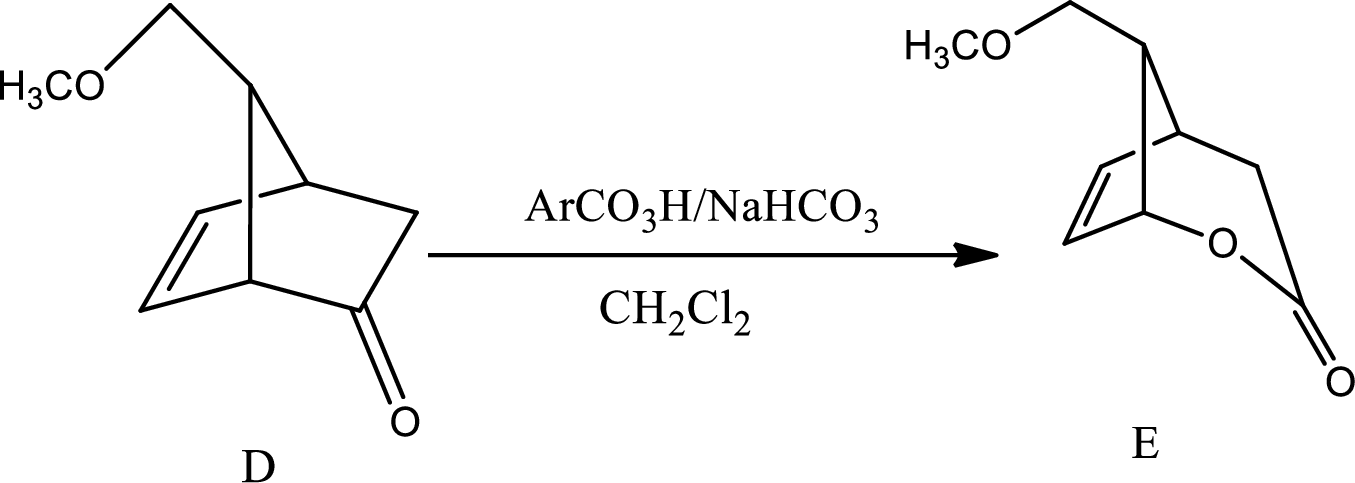
The mechanism is as follows,
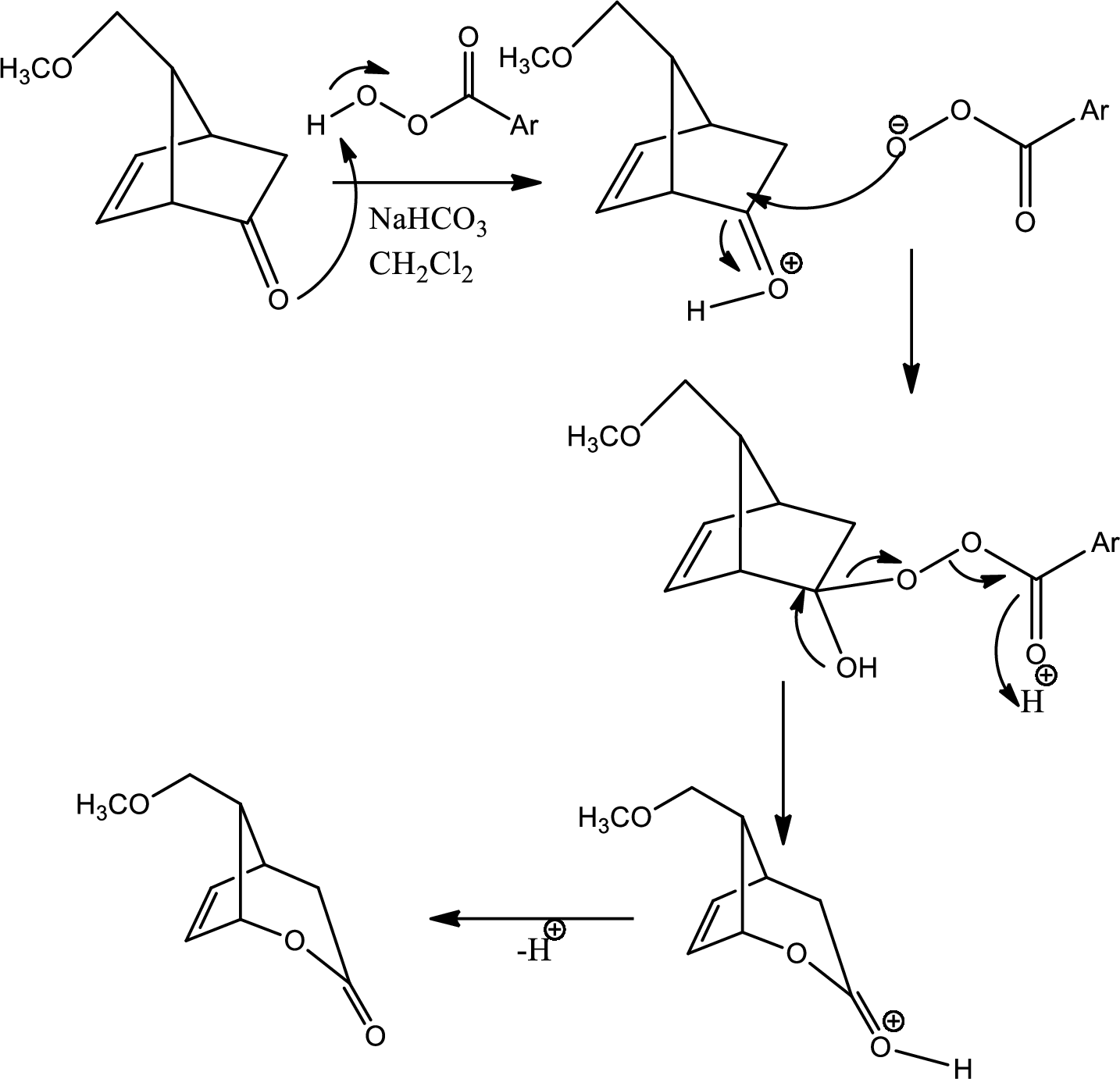
This is the pathway for D to E via Baeyer-Villiger oxidation. The process starts with acid base reaction followed by
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- ● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forwardThe aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forward
- ion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forward
- K Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

