
Interpretation:
The proper reactant and the proper coupling reaction pathway have to be suggested for the given
Concept Introduction:
Cross coupling:
A cross coupling reaction is defined as a reaction that creates a
In the case of palladium catalysed cross-coupling reactions the other metal or metalloids are commonly
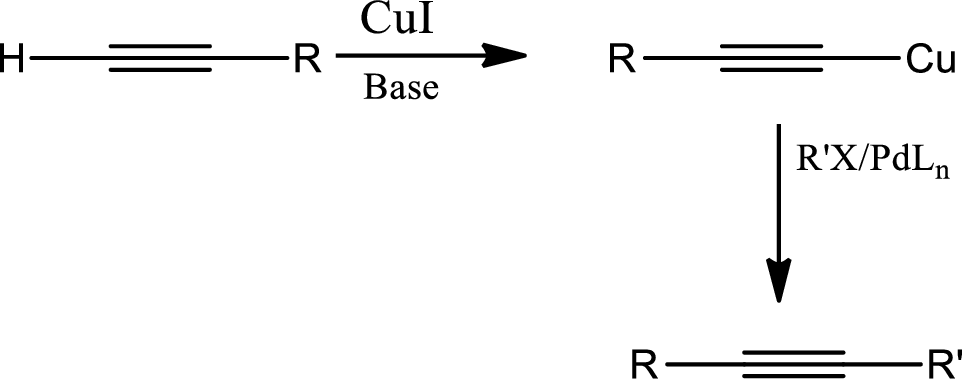
Sonogashira coupling:
The
Generalized reaction,
Suzuki coupling:
The Suzuki coupling uses a boron compound and an alkenyl, aryl or alkyl halide or triflate as the carbon sources with a palladium salt as a catalyst. The reaction is mainly used to form biaryls. The mechanism of the reaction starts with an oxidative addition followed by transmetallation in which the substituent on the borane replaces the ligand on the palladium concluding with the reductive elimination of the palladium to form the new carbon-carbon bond. The base may serve as a new labile ligand to palladium or it may activate the borane by coordination.
Generalized reaction,
Oxidative addition and ligand exchange,
Borane activation
Reaction,
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- Name an interesting derivative of barbituric acid, describing its structure.arrow_forwardBriefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward
- 4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forwardIndicate the importance of the indole ring. Find a representative example and list 5 structures.arrow_forwardΌΗ 1) V2 CO 3 or Nalt In منهarrow_forward
- 6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forwardConsider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forward
- Given Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forwardFor a certain gas-phase reaction at constant pressure, the equilibrium constant Kp is observed to double when the temperature increases from 300 K to 400 K. Calculate the enthalpy change of the reaction, Ah, using this information.arrow_forwardHydrogen bonding in water plays a key role in its physical properties. Assume that the energy required to break a hydrogen bond is approximately 8 kJ/mol. Consider a simplified two-state model where a "formed" hydrogen bond is in the ground state and a "broken" bond is in the excited state. Using this model: • Calculate the fraction of broken hydrogen bonds at T = 300 K, and also at T = 273 K and T = 373 K. • At what temperature would approximately 50% of the hydrogen bonds be broken? • What does your result imply about the accuracy or limitations of the two-state model in describing hydrogen bonding in water? Finally, applying your understanding: • Would you expect it to be easier or harder to vaporize water at higher temperatures? Why? If you were to hang wet laundry outside, would it dry more quickly on a warm summer day or on a cold winter day, assuming humidity is constant?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


