
(a)
Interpretation:
Reasonable precursors have to be suggested for the formation of compound I in the given problem.
Concept introduction:
Amide bond formation:
Amides, commonly known as acid amides are those compounds which have
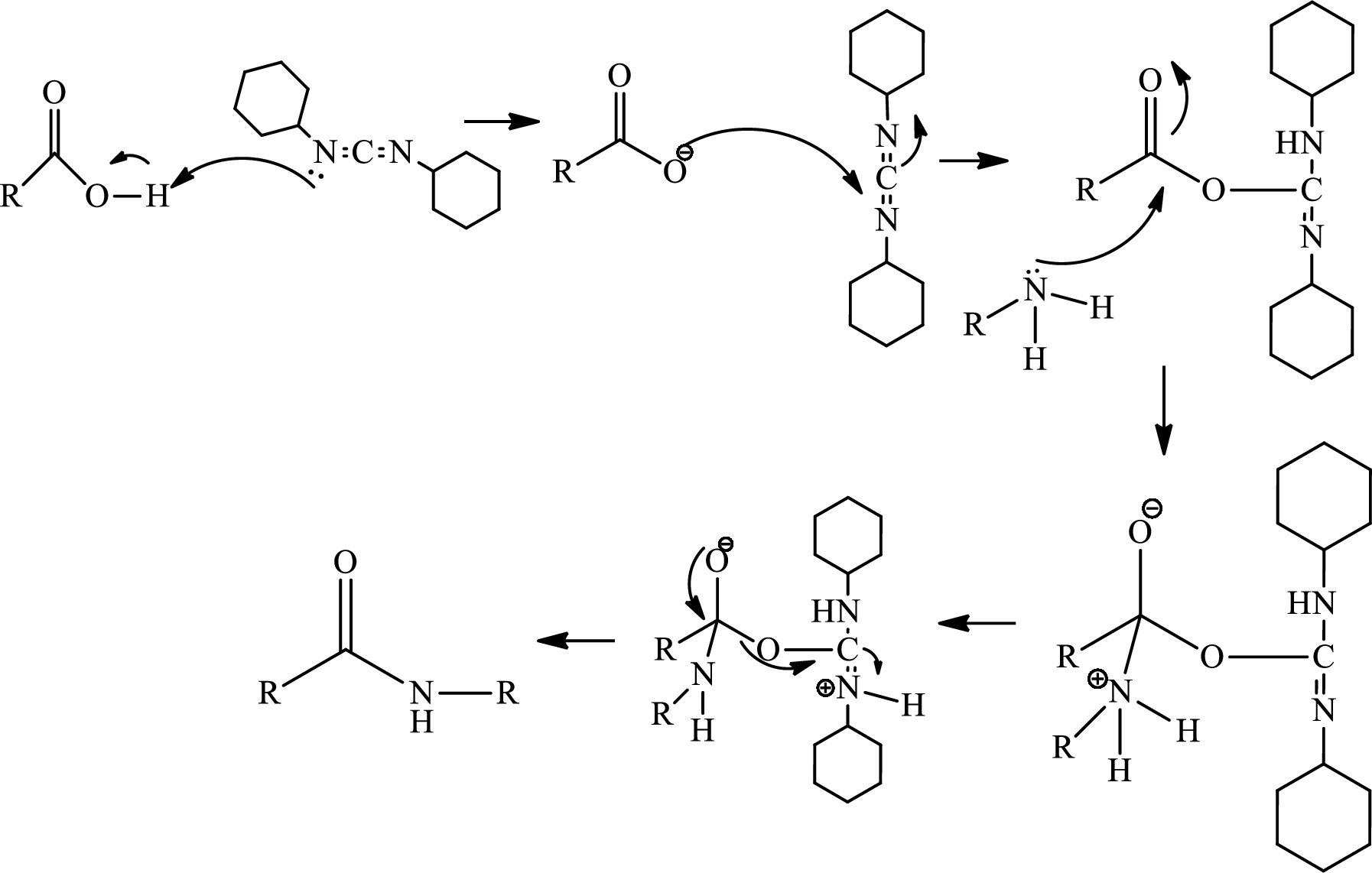
(b)
Interpretation:
Reagents for the reaction along with the mechanism have to be given for the conversion of compound I to II as given in the question.
Concept introduction:
Coupling reaction of aryl halides and phenols catalyzed by palladium and MOP types of ligands:
Palladium catalyzed coupling reactions of aryl halides and phenols are described employing the bulky and electron rich MOP type ligands. When
(c)
Interpretation:
The two isomers of compound II have to be shown.
Concept introduction:
Atropisomers:
Atropisomers are the stereoisomers arising because of the hindered rotation about a single bond where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.
(d)
Interpretation:
Suitable reagents have to be given for the transformation of compound II to III in the given problem.
Concept introduction:
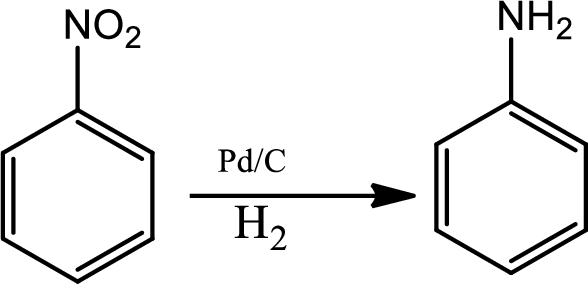
Reduction of nitro group:
Reduction of nitro group can be done by various reagents. In industrial scale reduction of nitrobenzene to anniline is a very common reaction. It is done by catalytic hydrogenation in the presence of palladium hydrogen.
Diazotisation reaction:
Diazonium salta are organic compounds having
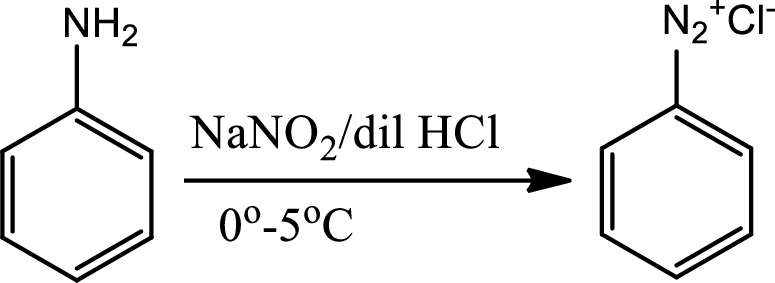
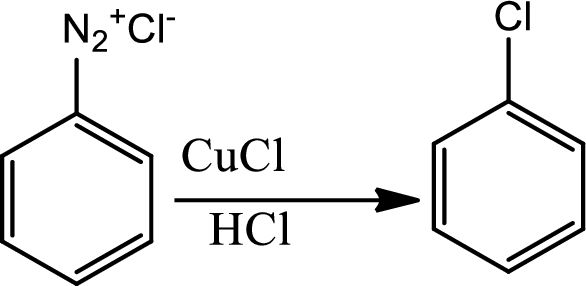
Sandmeyer reaction:
The Sandmeyer reaction is a
(e)
Interpretation:
The reagents and the fragment of the ring A that is useful for the conversion of compound III to IV have to be given.
Concept introduction:
Grignard reaction:
A grignard reagent is a chemical compound with the formula
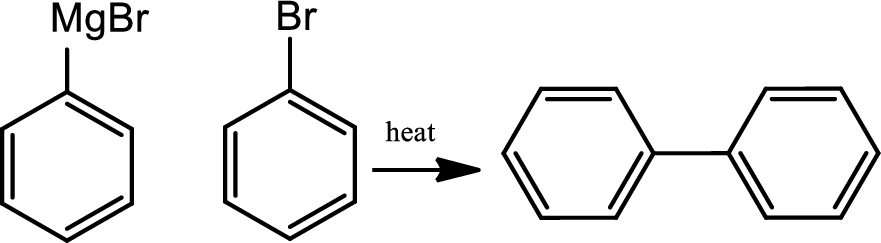
(f)
Interpretation:
Ring closure reaction of the deprotected free amino group has to be given along with the mechanism.
Concept introduction:
Amide bond formation:
Amides, commonly known as acid amides are those compounds which have
(g)
Interpretation:
The atropisomers have to be drawn and also it has to be shown that only one of them can be converted to vancomycin.
Concept introduction:
Atropisomers:
Atropisomers are the stereoisomers arising because of the hindered rotation about a single bond where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning




