
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 24, Problem 24.13P
Interpretation Introduction
Interpretation:
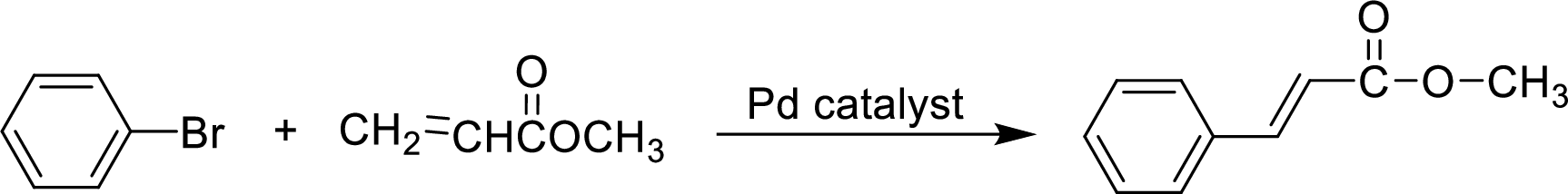
In the given reaction, the formation of the product and for the cis-stereochemistry of its ring junction has to be accounted.
Concept Introduction:
Heck reaction:
A palladium-catalyzed reaction of the carbon group of a haloalkene substituted for a hydrogen on double bonded carbon of an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
What is the name of the following compound?
SiMe3
K
Draw the starting structure that would lead to the major
product shown under the provided conditions.
Drawing
1. NaNH2
2. PhCH2Br
4 57°F
Sunny
Q Search
Chapter 24 Solutions
Organic Chemistry
Ch. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.4 - Prob. 24.3PCh. 24.5 - Show how the following compound can be prepared...Ch. 24.5 - Prob. 24.5PCh. 24.5 - Prob. 24.6PCh. 24.6 - Prob. 24.7PCh. 24 - Prob. 24.8PCh. 24 - Prob. 24.9PCh. 24 - Prob. 24.10P
Ch. 24 - Treatment of cyclohexene with iodobenzene under...Ch. 24 - Prob. 24.12PCh. 24 - Prob. 24.13PCh. 24 - The aryl diene undergoes sequential Heck reactions...Ch. 24 - Heck reactions take place with alkynes as well as...Ch. 24 - Prob. 24.16PCh. 24 - The following transformation involves a series of...Ch. 24 - Show the sequence of Heck reactions by which the...Ch. 24 - Prob. 24.19PCh. 24 - Write the steps that are critical in the following...Ch. 24 - Prob. 24.21PCh. 24 - Prob. 24.22PCh. 24 - Prob. 24.23PCh. 24 - Show how the following compound could be prepared...Ch. 24 - It is typically very difficult to do a...Ch. 24 - The compound eutypine is an antibacterial agent...Ch. 24 - Prob. 24.27PCh. 24 - Prob. 24.28PCh. 24 - Prob. 24.29PCh. 24 - Prob. 24.30PCh. 24 - Prob. 24.31PCh. 24 - Prob. 24.32PCh. 24 - Prob. 24.33PCh. 24 - The following transformation can be accomplished...Ch. 24 - Prob. 24.35PCh. 24 - Prob. 24.36PCh. 24 - Prob. 24.37PCh. 24 - Prob. 24.38PCh. 24 - E. J. Coreys 1964 total synthesis of...Ch. 24 - Prob. 24.40P
Knowledge Booster
Similar questions
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
