
Concept explainers
(a)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the
The block diagram to represent an overview of glycolysis is as follows:
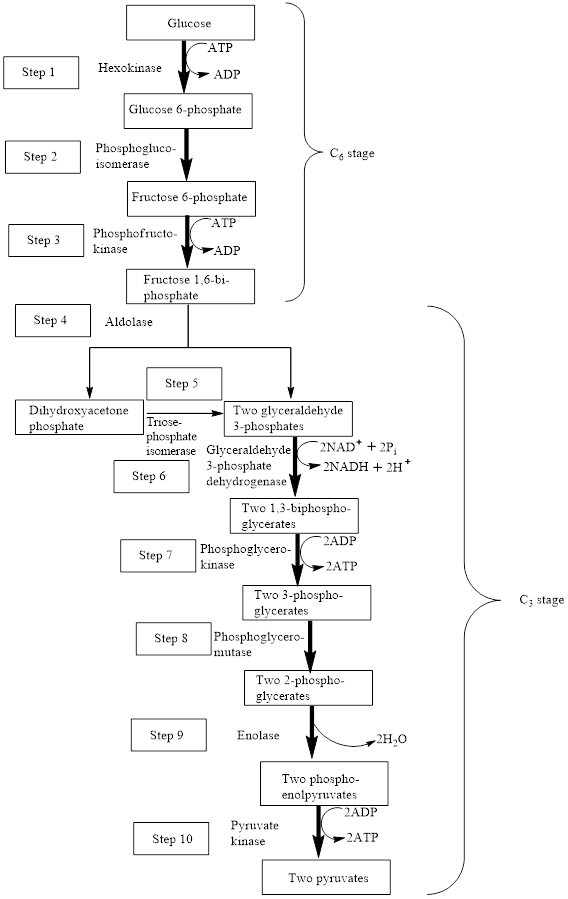
From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
The transfer of a phosphoryl group
(a)
Answer to Problem 24.19EP
The complete word equation is as follows:
Explanation of Solution
The first step in the glycolysis process is the phosphorylation of glucose using ATP. Glucose is converted to
In step 1, glucose is converted to
(b)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH reduced coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
(b)
Answer to Problem 24.19EP
The complete word equation is as follows:
Explanation of Solution
In step 9,
In step 9,
(c)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH reduced coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
A mutase enzyme catalyzes the isomerization reaction by shifting a functional group from one position to another position within a molecule.
(c)
Answer to Problem 24.19EP
The complete word equation is as follows:
Explanation of Solution
In step 8,
In step 8,
(d)
Interpretation: To identify the name of the substance in the following word equation.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH reduced coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
The transfer of a phosphoryl group
(d)
Answer to Problem 24.19EP
The complete word equation is as follows:
Explanation of Solution
The seventh step in the glycolysis process is the phosphorylation of
In step 7,
Want to see more full solutions like this?
Chapter 24 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





